Abstract
Background: Anterior fontanel is an integral element of an infant craniofacial system. There are six fontanels in the newborn skull, namely anterior, posterior, two mastoid, and two sphenoid fontanels. The anterior fontanel is the largest, prominent, and most important for clinical evaluation. Sex, race, genetics, gestational age, and region are the principal factors that influence anterior fontanel size. There exist inconclusive findings on the size of anterior fontanel in newborns. Therefore, this systematic review and meta-analysis aimed to determine the pooled mean size of anterior fontanel among term newborns and to identify the pooled mean difference of anterior fontanel size between males and females.
Methods: PubMed/Medline, Google Scholar, Science Direct, JBI Library, embase, and Cochrane Library databases were systematically searched. All essential data were extracted using a standardized data extraction format. The heterogeneity across studies was assessed using the Cochrane Q test statistic, I2 test statistic, and p-values. A fixed-effect model and random effect model were used to estimate the pooled mean size of anterior fontanel and the pooled mean difference between male newborns and female newborns, respectively. To deal with heterogeneity, sub-group analysis, meta-regression analysis, and sensitivity analysis were considered. JBI quality appraisal checklist was used to evaluate the quality of studies.
Results: In this meta-analysis, 8, 661 newborns were involved in twenty-six studies. Among studies, 13 conducted in Asia, 7 in Africa, 5 in America, and 1 in Europe. The pooled mean size of anterior fontanel was 2.58 cm (95% CI: 2.31, 2.85 cm). The pooled mean size of anterior fontanel for Asia, Africa, America, and Europe region was 2.49, 3.15, 2.35, and 2.01 cm, respectively. A statistically significant mean difference was detected between male and female newborns (D + L pooled MD = 0.15 cm, 95% CI: 0.02, 0.29 cm).
Conclusion: The pooled estimate of this review does provide the mean value of the anterior fontanel size in the newborns. There was a statistically significant mean fontanel size difference between male and female newborns. Therefore, male newborns had a significantly larger mean size than female newborns.
Background
Fontanels are defined as gaps happening when more than two cranial bones are juxtaposed [1–3]. Narrow ridges of fibrous connective tissue, which is called sutures, join the flat bones of the skull [2–5]. Anterior, posterior, two mastoid, and two sphenoid fontanels can be identified in the newborn skull [4–10]. The largest rhomboid anterior fontanel is situated between the two frontal and two parietal bones. This fontanel is the prominent and most important for clinical evaluation [1–3, 5–10]. The mean time of anterior fontanel closure is eighteen months but usually closes by twelve months [5–7, 10]. A place where two or more sutures meet is called the fontanel [10]. The sutures and fontanels in the normal skull, especially anterior fontanel, allow the growth of the brain relative to skull bone growth [1, 2, 9, 10]. Besides, the bones of the skull overlap each other during the labor time for successful delivery; however, the molding process of the skull usually resolved after three to five days of birth [1, 2, 7, 8]. The anterior fontanel is an integral element of an infant craniofacial system [8–10]. The diagnosis of an abnormal fontanel requires an understanding of the wide variation of normal fontanel [7–10]. Knowledge of anterior fontanel size is crucial to identify many disorders. A very small size of the anterior fontanel (or early fontanel closure at birth) can be associated with craniosynostosis and abnormal brain development [8–10]. The large size of the anterior fontanel can be associated with multiple diseases. Of them, skeletal disorders, chromosomal defects and dysmorphogenesis syndromes, endocrine disorders, drug and toxin exposure, fetal hydantoin syndrome, aminopterin induced malformations, congenital infections (rubella and syphilis, for example), and aluminum toxicity [1, 5, 8, 9]. Furthermore, increased intracranial pressure is the most common cause of bulging or delayed closure of the anterior fontanel [8]. A sunken anterior fontanel is the sign of dehydration [1, 8–10]. Anterior fontanel size has been utilized as evidence of altered intracranial pressure, an index of the rate of development, and ossification of the calvarium [11]. It is also an indicator of various medical disorders and abnormal skeletal morphogenesis [1, 2, 7, 11, 12]. The variation in size, shape, and closure time is a key feature of anterior fontanel [8, 11]. Significantly, sex, race, gestational age, genetics, regions, and nutrition are the principal factors that influence anterior fontanel size [10–19]. The developmental anatomy of anterior fontanel is also affected by the rate of brain growth, dural attachment, suture development, and osteogenesis [11]. Incredibly, the difference in the mean size of the anterior fontanel between sexes is inconsistent across different studies. At birth, studies conducted elsewhere reported discrepancies in the mean size of anterior fontanel between sexes. Some of the studies reported that the mean size of the male newborns is significantly larger than the female newborns [7–10, 16, 20, 21]. However, some other reports did not show a significant difference in anterior fontanel size between male and female newborns [11, 13, 15, 22–25].
In different parts of the globe, several studies have been conducted to determine the mean size of the anterior fontanel. However, the studies were inconclusive and there is no concrete evidence established at the global level that pooled the average value of anterior fontanel size. Furthermore, despite there are fragmented studies (presented various local or country-level reference range between sexes) performed across the globe that explore the mean difference of anterior fontanel size between male and female newborns, the findings reported from these studies were controversial and inconclusive. For instance, in most studies, male newborns had significantly larger anterior fontanel size than female newborns. In others, the differences in anterior fontanel size between sexes were non-significant.
Given abnormal fontanel can indicate a serious medical condition [1, 8, 9], it is important to understand the pooled mean size of anterior fontanel and the pooled mean difference of anterior fontanel size between male and female newborns. These findings, pooled average value and pooled mean difference between sexes, provide valuable information to Pediatricians, Anatomists, Neurosurgeons, Neuroradiologists, and other Medical personnel for newborn examination. Therefore, this systematic review and meta-analysis aimed to determine the pooled mean size of anterior fontanel among term newborns and to identify the pooled mean difference of anterior fontanel size between male and female newborns.
Methods
Searching Strategies
To avoid duplication, the presence of systematic review and meta-analysis on the topic of interest were checked on different databases (DARE database, Cochrane Library, and JBI Library, for example). PubMed/Medline, Cochrane Library, JBI Library, CINAHL, Google Scholar, Science Direct, Web of Science, and embase databases were systematically searched for relevant studies. Gray literature and other sources were retrieved using Google and Google Scholar searches. Besides, reference lists (bibliographies) of identified studies were checked for the presence of additional studies. Sources including the websites of local libraries were also retrieved. The primary search was conducted in the PubMed database. The search was conducted using the following search strategies (“Anterior fontanelle size” OR “anterior fontanel size” [MeSH Terms] OR “fontanel* size” OR “average size of fontanel*” OR “mean size of fontanel” OR “size of anterior fontanel” [MeSH Terms] OR “anterior fontanelle*” OR “measurement of anterior fontanelle” OR Fontanelles*[MeSH Terms]) AND (“term newborn*” [MeSH Terms] OR “newborn*” OR “term neonate” OR “term infant” OR “term children”). Core search terms and phrases used in different databases were “anterior fontanel size” and “term newborns”.
Study Inclusion and Exclusion Criteria
The inclusion criteria for this systematic review and meta-analysis were published and unpublished full-text articles in the English language at any time and design. Furthermore, it was included articles referring to healthy term newborns, up to three days of life, with normal birth weight and that reported a mean and standard deviation for anterior fontanel.
It was excluded articles with reference to premature newborns, post-term newborns, low birth weight, macrosomic newborns, with known pathology, multiple pregnancies, and image-based studies.
Study Outcome and Covariates
The primary outcome of this review was the pooled mean size of anterior fontanel among term newborns. The second outcome was to compare the mean difference of anterior fontanel size between male and female newborns.
The Methods for Assessing the Size of the Anterior Fontanel
In different nations, there are various methods for assessing the size of the anterior fontanel (traditional method, Area, Oblique diameter, for instance) described as simple clinical methods of measuring anterior fontanel size [2, 7–10]. Many researchers are interested to use the most popular method of Popich and Smith, known as the traditional method [12]. This method is the simplest, practical, and acceptable in clinical settings. To circumvent the problem of the fontanel ended and the suture began, the extent of the anterior fontanel was determined by inserting the index finger in turn into each of the four vertices and a small circular dot was marked with washable ink on the skin immediately distal to the finger. A piece of white paper was firmly pressed over the fontanel so that the four dots were transferred onto the paper [26]. The distance between the anterior and posterior points and between the transversal points was measured and recorded with a fresh ruler. The average of anterior-posterior dimension and transverse dimension was considered as the size of anterior fontanel [10, 12].
Study Quality Assessment
The Joanna Briggs Institute (JBI) quality appraisal checklist was considered to assess the quality of each study [27]. Two reviewers independently assessed the quality of each study using the tool. Disagreements between reviewers that arise during criticizing the quality of the study were negotiated based on the evidence-based discussions. The JBI critical appraisal checklist for cross-sectional studies was adapted. It contains eight items that are listed from “the criteria for inclusion in the sample clearly defined” to “the appropriateness of the statistical analysis” (Additional file 1). In the end, the study was considered low risk if the study scored fifty and above percent of all quality assessment items of the study design.
Data Extraction Strategy and Study Selection
After retrieving all studies from the databases, citations were imported into the bibliographic software, Endnote Version 7 Software, to remove the duplicate studies. After the removal of duplicate articles, the remaining studies were screened based on title and abstract for possible inclusion. Full-text articles were deeply reviewed for the entirely to determine the final included article. Two reviewers using a standardized data extraction template extracted all essential data independently. The data abstraction format included primary author, publication year, sample size, country of the study, study design, sex of newborns, mean size of the anterior fontanel, standard deviation, methods, measuring instrument used, male sex (sample size, mean, standard deviation, p-value), female sex (sample size, mean, standard deviation), and other parameters.
Data Synthesis and Presentation
The data analyses were conducted using STATA version 14.1 Statistical Software. The data were extracted in Microsoft Excel and exported into STATA for further analysis.
Statistical Analysis
Meta-Analysis
The heterogeneity across the studies was assessed using the Cochrane Q test statistic (chi-square), I2 test statistic, and p-values. The heterogeneity was declared as low, moderate, or high when I2 test statistics results were 25%, 50%, and 75%, respectively [28]. In the case of estimating the pooled mean size of anterior fontanel, there was no statistically significant heterogeneity among studies (I2 = 0.0%, p-value = 0.943), therefore, a fixed-effect model was used to estimate the pooled mean size of anterior fontanel [29]. However, in estimating the mean difference between sexes, heterogeneity was detected (I2 = 85.5%, p-value<0.001). Due to the presence of heterogeneity, a random-effect model (then, sub-group analysis, meta-regression analysis, and sensitivity analysis were considered) was used. A sensitivity analysis was done to observe the influence of a single study on the overall estimation of meta-analysis. Meta-regression analysis was accounted for to identify the source of heterogeneity across the studies. The forest plot and Galbraith plot were used to visualize the presence of heterogeneity among studies. Furthermore, a meta-cumulative analysis was done to display the pattern of effects and the significance of cumulative effect over the publication years. To observe the random variations in time sequence among the studies, a time-trend analysis was undertaken. In all cases, a p-value less than or equal to 0.05 was considered statistically significant.
Assessment of Risk of Bias in Studies
The publication bias was checked by Egger’s regression test and Begg’s test [30, 31]. Statistically significant publication bias was considered if a p-value becomes ≤0.05. Egger’s plot was used to visualize the publication bias.
Results
Accessed Studies
The reports of this systematic review and meta-analysis were presented based on the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statements [32] (Additional file 2). A total of 372 articles were initially retrieved regarding anterior fontanel size through PubMed, Google Scholar, Science Direct, Cochrane Library, JBI Library, Medline, Embase, and others. Of these, 93 were excluded due to duplicated articles. From the remaining 279 articles, 220 articles were excluded after reviewing its titles and abstracts because titles were found irrelevant for this study. The rest 59 articles were screened for full-texts and 29 were excluded due to the outcome of interest. Therefore, 30 full-text articles were assessed for eligibility based on the pre-determined criteria (four excluded [33–36]). Finally, 26 studies were fulfilled the eligibility criteria and included in the systematic review and meta-analysis (Figure 1).
FIGURE 1
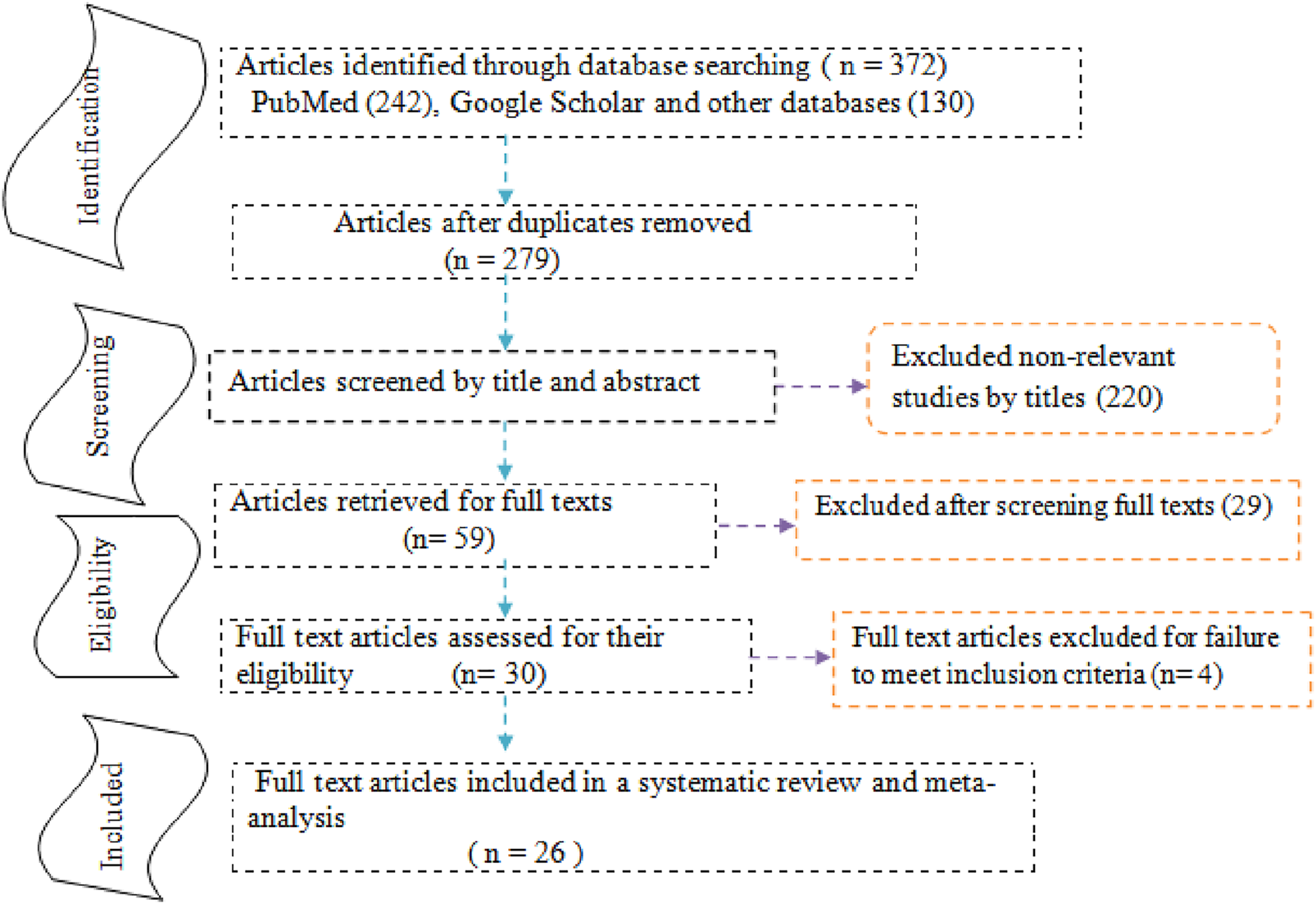
Study selection flow diagram; a figure adapted from the PRISMA group statement in estimating anterior fontanel size.
Characteristics of the Original Studies
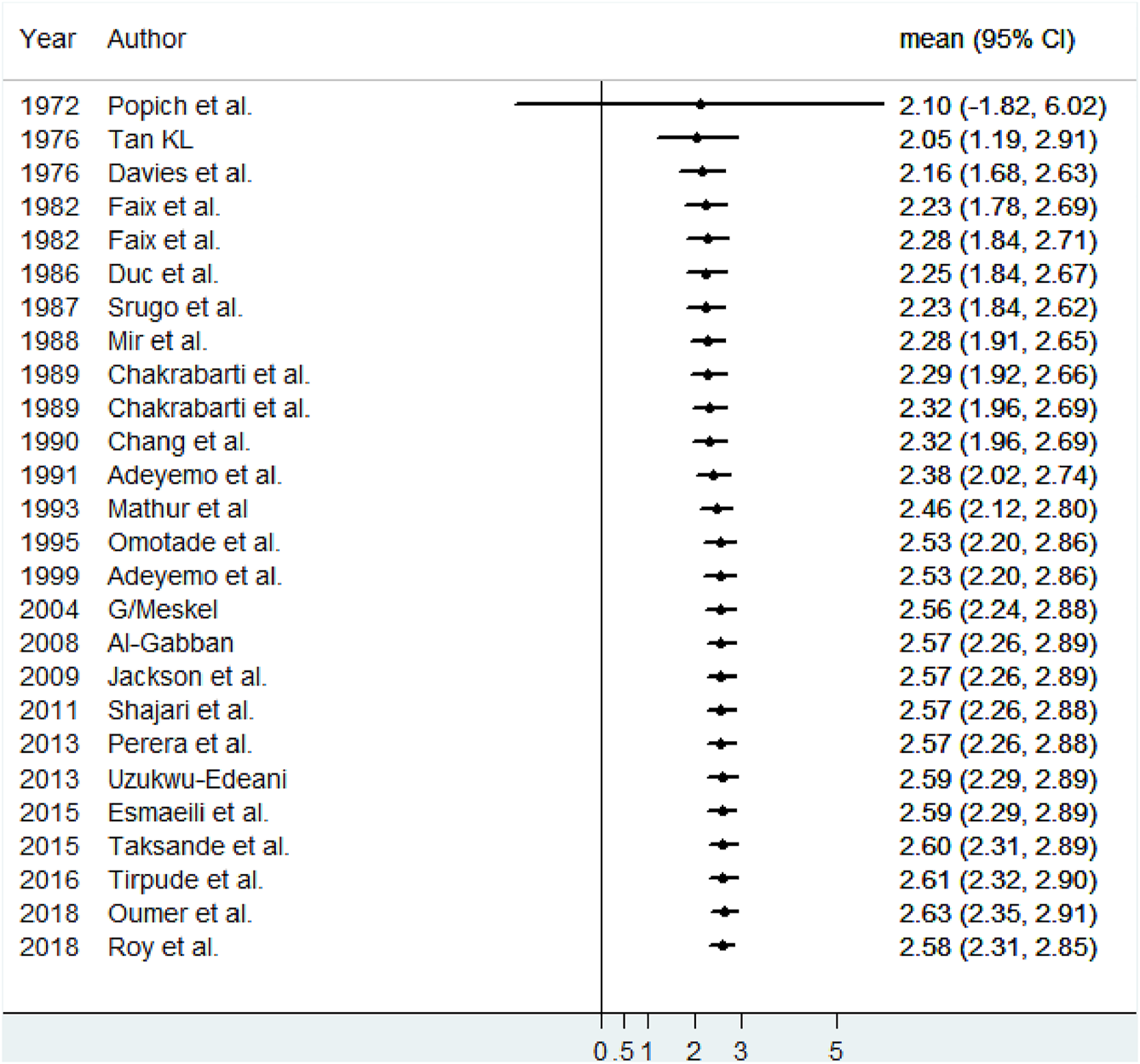
All included studies had used a cross-sectional study design [7–21, 23–26, 37–41]. The studies included a variable number of newborns, ranging from 33 to 2, 215 newborns [21, 26]. The largest study was carried out in Sri Lankan. From all studies, thirteen conducted in Asia [7, 8, 11, 14, 17, 19, 21, 23, 24, 38, 40, 41], seven in Africa [9, 10, 13, 20, 25, 37, 39], five in America [12, 15, 16, 26], and one in Europe [18]. The total size included in this review were 8, 661 newborns. All studies were published in the year between 1972 and 2018. Two articles reported two mean values for two different racial groups (Black American and White American and Hilly and non-hilly Indian). Thus, we included them separately in the analysis [15, 17] (Table 1).
TABLE 1
| First author | Year | Country | Study design | Sample size | Mean | ±S.D. | Measuring instrument | Method | Quality status |
|---|---|---|---|---|---|---|---|---|---|
| Uzukwu-edeani [13] | 2013 | Nigeria | CS | 269 | 2.97 | 0.71 | Steel tape | Trad | Low risk |
| Faix et al. [15] | 1982 | America | CS | 293 | 3.08 | 0.8 | Paper tape | Trad | Low risk |
| Mathur et al. [14] | 1993 | India | CS | 445 | 3.37 | 0.61 | SC | Trad | Low risk |
| Chakrabarti et al. [17] | 1989 | India | CS | 110 | 3.35 | 1.07 | Tape | Trad | Low risk |
| Chakrabarti et al. [17] | 1989 | India | CS | 130 | 3.8 | 1.95 | Tape | Trad | Low risk |
| Tirpude et al. [11] | 2016 | India | CS | 698 | 4.24 | 2.21 | VC | Trad | Low risk |
| Mir et al. [20] | 1988 | Libya | CS | 200 | 2.72 | 0.63 | Steel tape | Trad | Low risk |
| Faix et al. [15] | 1982 | America | CS | 73 | 2.67 | 0.7 | Paper tape | Trad | Low risk |
| Chang et al. [19] | 1990 | China | CS | 79 | 2.67 | 5.75 | Steel tape | Trad | Low risk |
| Omotade et al. [25] | 1995 | Nigeria | CS | 337 | 3.4 | 0.6 | Steel tape | Trad | Low risk |
| Adeyemo et al. [37] | 1991 | Nigeria | CS | 200 | 4 | 1 | Tape | Trad | Low risk |
| Shajari et al. [7] | 2011 | Iran | CS | 400 | 2.54 | 1.33 | Paper tape | Trad | Low risk |
| Esmaeili et al. [8] | 2015 | Iran | CS | 208 | 2.55 | 1.92 | Steel ruler | Trad | Low risk |
| Perera et al. [21] | 2013 | SriLanka | CS | 2215 | 2.55 | 0.92 | Plastic tape | Trad | Low risk |
| Srugo et al. [38] | 1987 | Israel | CS | 303 | 2.06 | 0.6 | Tape | Trad | Low risk |
| Popich et al. [12] | 1972 | America | CS | 201 | 2.1 | 2 | Steel tape | Trad | Low risk |
| Davies et al. [26] | 1976 | America | CS | 33 | 220.2 | 28.6 | Steel tape | Area | Low risk |
| Jackson et al. [16] | 2009 | Hispanic | CS | 170 | 2.25 | 7.9 | DC | Trad | Low risk |
| Duc et al. [18] | 1986 | Switzerland | CS | 111 | 2.01 | 0.72 | Caliper | Oblique | Low risk |
| G/Meskel [9] | 2004 | Ethiopia | CS | 78 | 3.35 | 0.94 | Ruler | Trad | Low risk |
| Oumer et al. [10] | 2018 | Ethiopia | CS | 384 | 3 | 0.62 | Ruler | Trad | Low risk |
| Adeyemo et al. [39] | 1999 | Nigeria | CS | 250 | 3.9 | 9.65 | Ruler | Trad | Low risk |
| Roy et al. [23] | 2018 | India | CS | 745 | 2.08 | 0.45 | Ruler | Trad | Low risk |
| Al-gabban [24] | 2008 | Iraq | CS | 200 | 2.79 | 0.71 | Tape | Trad | Low risk |
| Taksande et al. [40] | 2015 | India | CS | 469 | 2.76 | 0.55 | Tape | Trad | Low risk |
| Tan KL [41] | 1976 | China | CS | 60 | 2.05 | 0.45 | Steel tape | Elsasser | Low risk |
The characteristics of original studies included in meta-analysis for mean size estimation of anterior fontanel, 2020.
CS, cross-sectional; Trad, traditional; VC, vernier caliper; DC, digital caliper; SC, sliding caliper; S.D., standard deviation.
Concerning the quality of studies, all included articles were assessed through the JBI quality appraisal criteria. The quality score of the included studies was ranged between fifty percent and ninety percent. Therefore, no studies were included that had considerable risk in the present review (Table 1).
The Mean Size of the Anterior Fontanel
In this meta-analysis, a significant heterogeneity was not found (effect size attributable to heterogeneity (I2) = 0.0%, p-value = 0.943, heterogeneity chi-square = 14.9). As a result, a fixed-effect model was applied to calculate the pooled mean size of anterior fontanel. Therefore, the pooled mean size of anterior fontanel was 2.58 cm (95% CI: 2.31, 2.85 cm) (Figure 2).
FIGURE 2
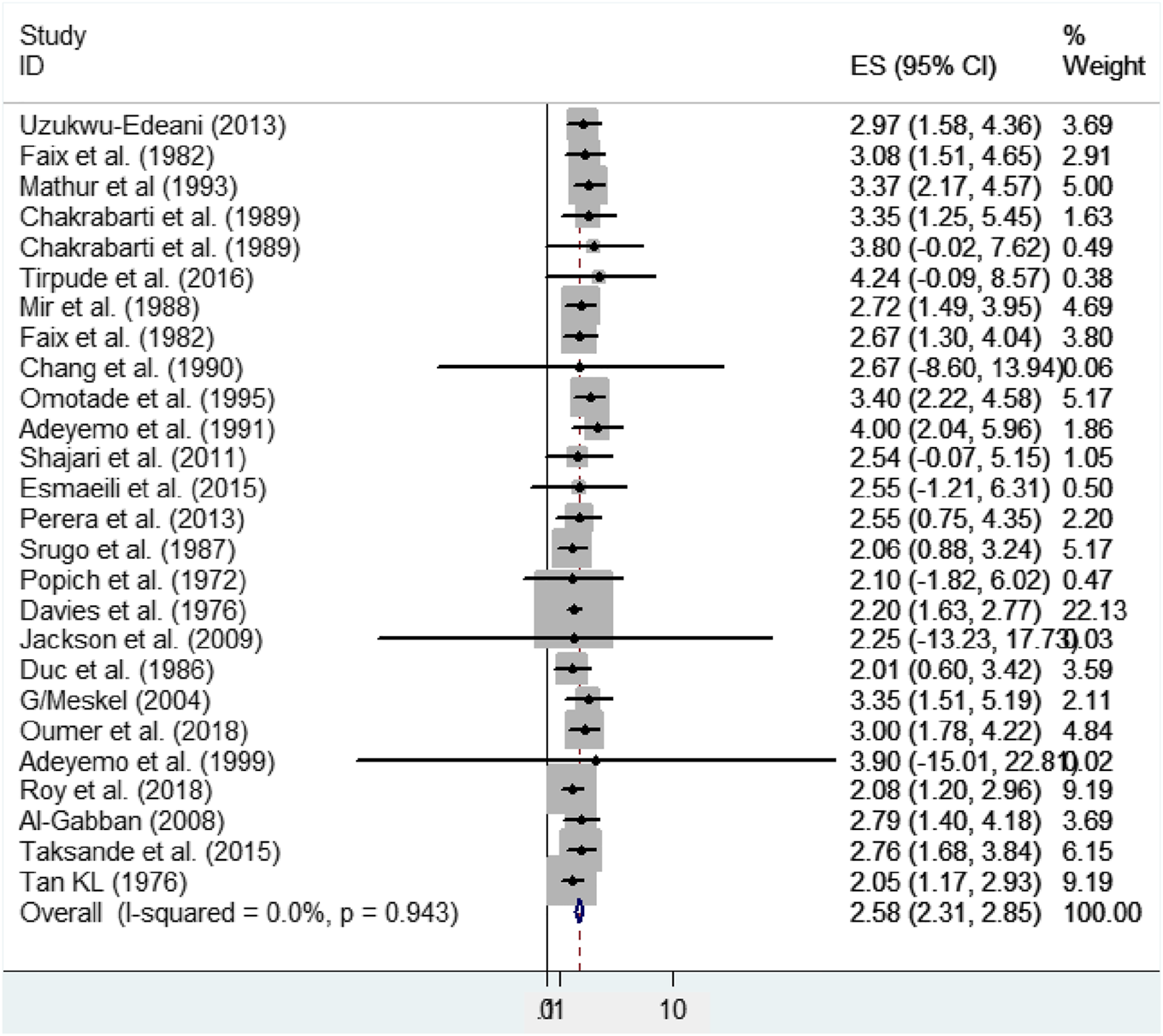
Forest plot showing the pooled mean size of anterior fontanel, 2020.
Graphically, the Galbraith plot, outlined all studies based on their country, showed that there is no variability between the studies in the mean size of the anterior fontanel because studies are located within its confidence interval limits (Figure 3).
FIGURE 3
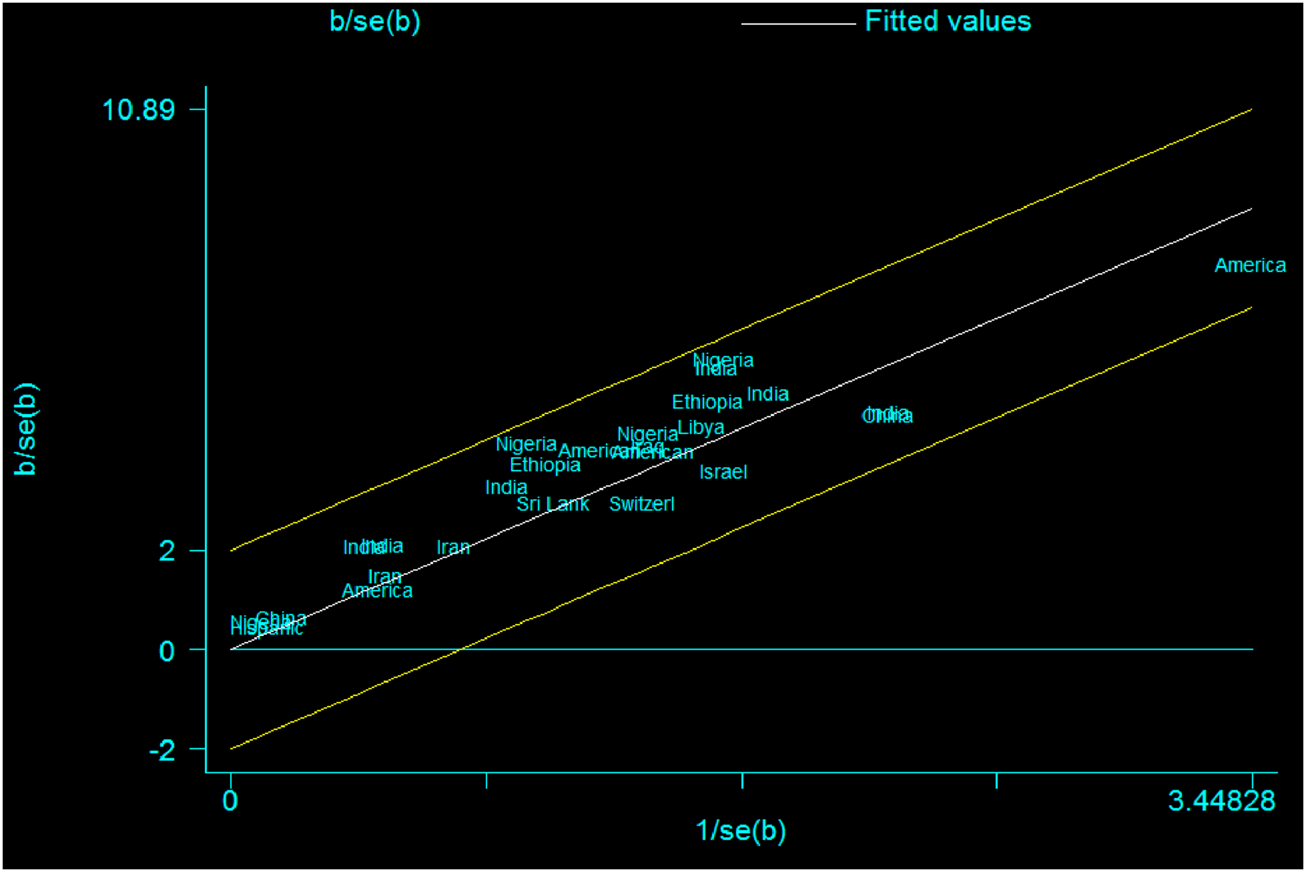
Galbraith plot showing the variability of individual mean size of the anterior fontanel by study country, 2020.
Based on the study region, the pooled mean size of anterior fontanel for Asia, Africa, United States, and Europe region was 2.49, 3.15, 2.35, and 2.01 cm, respectively (Table 2).
TABLE 2
| S. No | Regions | Mean size of anterior fontanel (95%CI) | I-squared, p-value | p-value |
|---|---|---|---|---|
| 1 | Africa | 3.15 (2.58, 3.71) | 0.0%, 0.957 | <0.001 |
| 2 | America | 2.35 (1.85, 2.84) | 0.0%, 0.857 | <0.001 |
| 3 | Asia | 2.49 (2.09, 2.89) | 0.0%, 0.857 | <0.001 |
| 4 | Europe | 2.01 (0.60, 3.42) | –, – | = 0.005 |
| Total | I-V pooled | 2.58 (2.31, 2.85) | 0.0%, 0.943 | <0.001 |
The pooled mean size of anterior fontanel according to the region of the studies, 2020.
Based on the study period, the pooled mean size of anterior fontanel for the year between 2011 and 2018 was 2.60 cm (95% CI: 2.09, 3.10; I2 = 0.0%, p-value = 0.91), for between 2001 and 2010 was 2.99 cm (95% CI: 1.88, 4.10; I2 = 0.0%, p-value = 0.89), for between 1991 and 2000 was 3.48 cm (95% CI: 2.71, 4.25; I2 = 0.0%, p-value = 0.96), for between 1981 and 1990 was 2.56 cm (95% CI: 1.99, 3.13; I2 = 0.0%, p-value = 0.91), and for between 1971 and 1980 was 2.16 cm (95% CI: 1.68, 2.63; I2 = 0.0%, p-value = 0.96).
This review described the cumulative effect (using meta-cumulative analysis) of the mean size of anterior fontanel from 1972 (2.10) to 2018 (2.58). Except for the first year, the cumulative effects of all studies were significant (Figure 4).
FIGURE 4
Meta-cumulative analysis showing the cumulative effect of the mean size of anterior fontanel, 2020.
The time-trend analysis showed the relationship between the mean value of anterior fontanel and publication year from 1972 (2.1) to 2018 (2.08) (Figure 5).
FIGURE 5
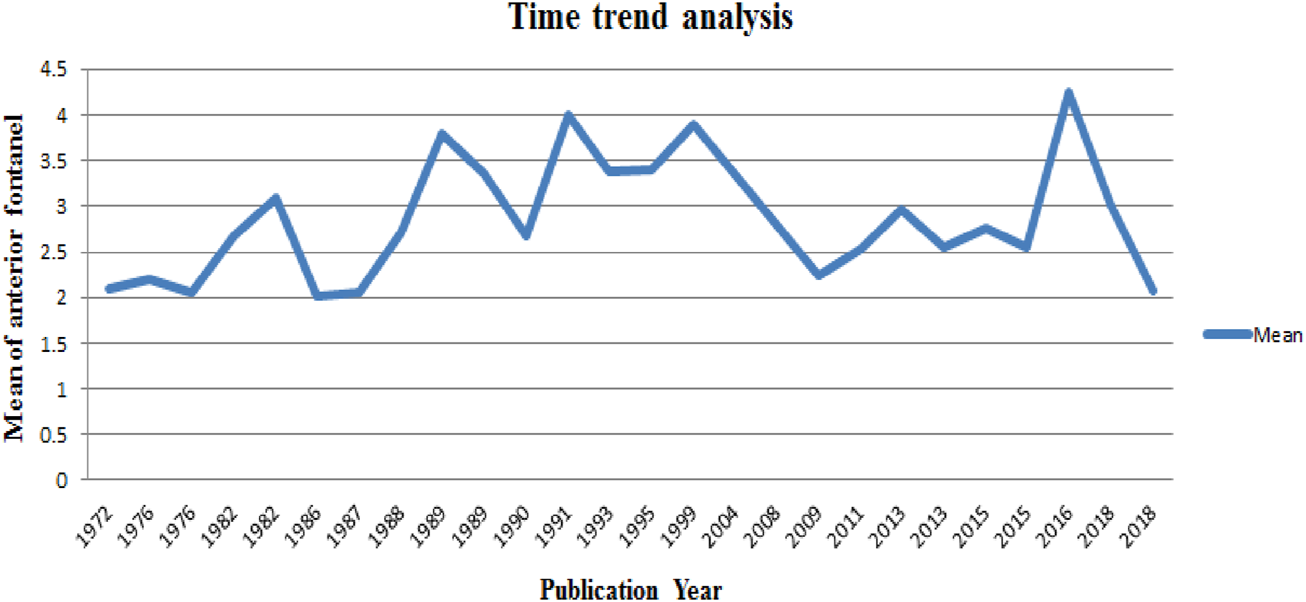
Time trend analysis of the mean value of anterior fontanel in relation to publication year, 2020.
Mean Difference of Anterior Fontanel Size Between Male and Female Newborns
From all studies included in this meta-analysis, twelve original studies [7–11, 13, 18–21, 23, 24] were considered to compare the mean difference of anterior fontanel size between male and female newborns. Seven studies were conducted from Asia [7, 8, 11, 19, 21, 23, 24], four in Africa [9, 10, 13, 20], and one in Europe [18]. The sample size, mean, standard deviation for both males and females were described elsewhere (Table 3).
TABLE 3
| First author | Year | Country | Male | Female | p-value | Region | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SS | Mean | S.D. | SS | Mean | S.D. | |||||
| Uzukwu-edeani | 2013 | Nigeria | 143 | 2.97 | 0.67 | 126 | 2.98 | 0.75 | 0.89 | Africa |
| Tirpude et al | 2016 | India | 352 | 4.48 | 2.26 | 346 | 4.02 | 2.2 | – | Asia |
| Mir et al | 1988 | Libya | 100 | 2.92 | 0.51 | 100 | 2.51 | 0.74 | 0.0001 | Africa |
| Chang et al | 1990 | China | 36 | 27.2 | 5 | 43 | 26.2 | 6.5 | – | Asia |
| Shajari et al | 2011 | Iran | 220 | 2.67 | 1.32 | 180 | 2.37 | 1.32 | 0.023 | Asia |
| Esmaeili et al | 2015 | Iran | 110 | 2.39 | 0.86 | 98 | 2.73 | 1.02 | 0.01 | Asia |
| Perera et al | 2013 | SriLanka | 1088 | 2.57 | 0.92 | 1127 | 2.52 | 0.92 | 0.07 | Asia |
| Duc et al | 1986 | Switzerland | 56 | 19.3 | 6.6 | 55 | 20.9 | 7.9 | – | Europe |
| G/Meskel | 2004 | Ethiopia | 40 | 3.53 | 1 | 38 | 3.19 | 0.85 | 0.11 | Africa |
| Oumer et al | 2018 | Ethiopia | 206 | 3.1 | 0.66 | 178 | 2.88 | 0.57 | 0.0001 | Africa |
| Roy et al | 2018 | ndia | 547 | 2.03 | 0.54 | 463 | 2.12 | 0.55 | 0.58 | Asia |
| Al-gabban | 2008 | Iraq | 100 | 2.99 | 0.73 | 100 | 2.58 | 0.65 | 0.004 | Asia |
Descriptive summary of studies included for meta-analysis of mean size difference of anterior fontanel, 2020.
SS, sample size; S.D., standard deviation.
Using meta-analysis, significant heterogeneity was found in estimating the mean difference (MD) between male and female newborns (I2 = 85.5%, p-value<0.001). Consequently, a random-effect model was applied to determine the pooled mean difference of anterior fontanel size (D + L pooled MD: 0.15, 95% CI: 0.02, 0.29). Therefore, male newborns had 0.15 times significantly larger mean fontanel size than female newborns (p-value = 0.03) (Figure 6).
FIGURE 6
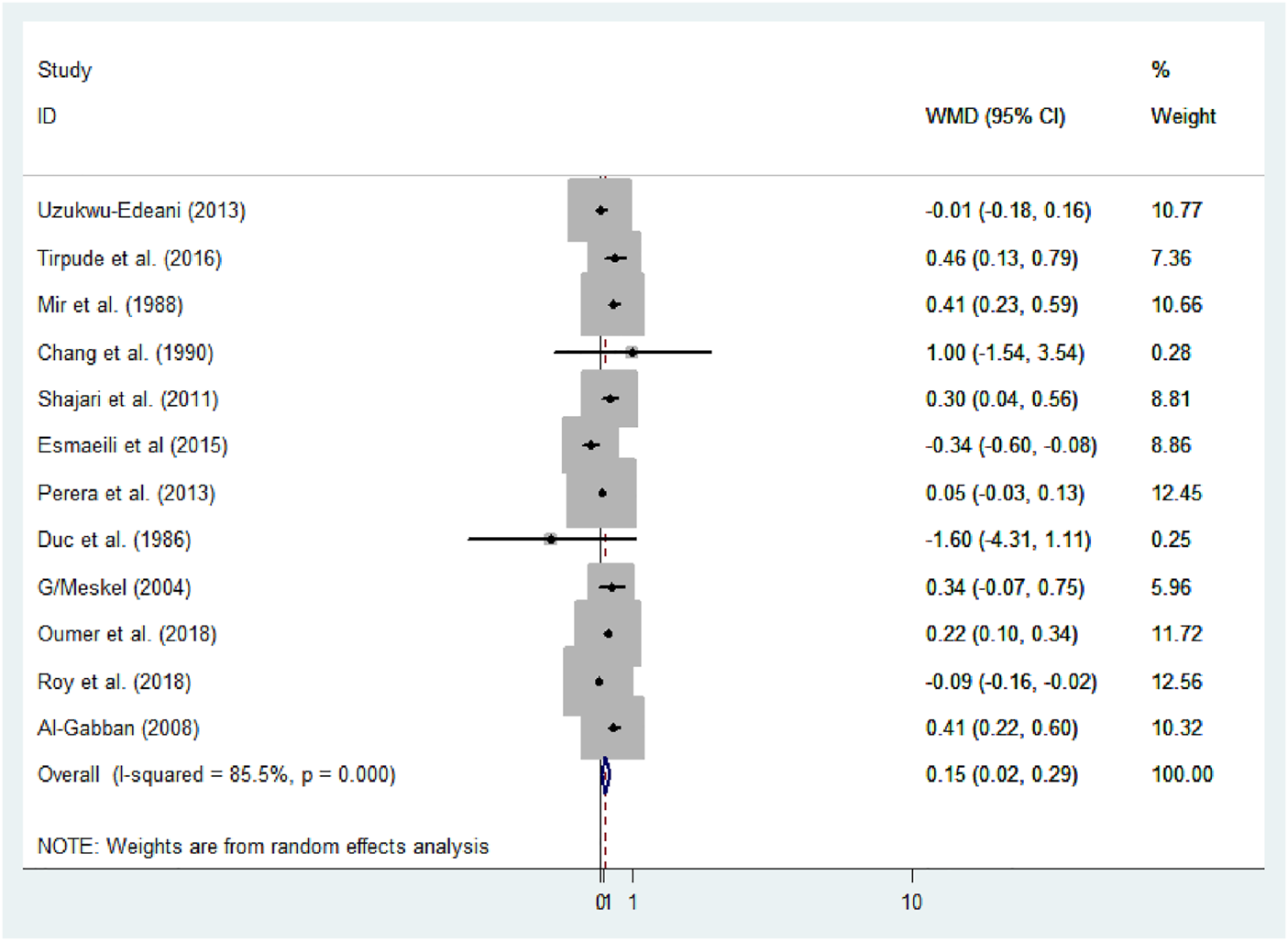
Forest plot showing mean difference of anterior fontanel size, 2020.
The Der Simonian and Laird’s (D + L) pooled prevalence method was considered because the Inverse variance method (I-V) can lead to unreliable estimates. The I-V method assumes that all heterogeneity can be attributed due to the covariates. This assumption may lead to an exaggerated type I error in the presence of residual or unexplained heterogeneity. Unstandardized mean difference (WMD) was used to estimate the effect because all studies were used the same measurement scale, centimeters.
Subgroup analysis based on the region, method, measuring instruments, and the study period was performed to detect the variation in mean difference across the studies. Based on the region, the high mean difference in anterior fontanel size was detected in Africa 0.22 cm (95% CI: 0.04, 0.41) (Figure 7). There was a significant variation in the mean difference of anterior fontanel between study regions (p-value<0.001). In the subgroup analysis of methods, there is significant heterogeneity between the traditional and oblique diameter methods (p-value<0.001). Because only one study used oblique diameter, it is difficult to predict the magnitude (Figure 8). From measuring instruments, steel ruler (p-value<0.001) and plastic ruler or tape (p-value<0.001) contribute to the significant variability of mean difference among studies. A statistically significant high mean difference in anterior fontanel size was detected in measuring plastic ruler or tape 0.17 cm (95% CI: 0.02, 0.33) (Figure 9). Based on study period, the mean difference of anterior fontanel size for the year after 2010 was 0.06 cm (95% CI: 0.07, 0.20; I2 = 84.9%, p-value<0.001), between 2001 and 2010 was 0.40 cm (95% CI: 0.22, 0.57; I2 = 0.00, p-value = 0.76), and between 1980 and 1990 was 0.32 cm (95% CI: 0.38, 0.03; I2 = 13.6, p-value = 0.31).
FIGURE 7
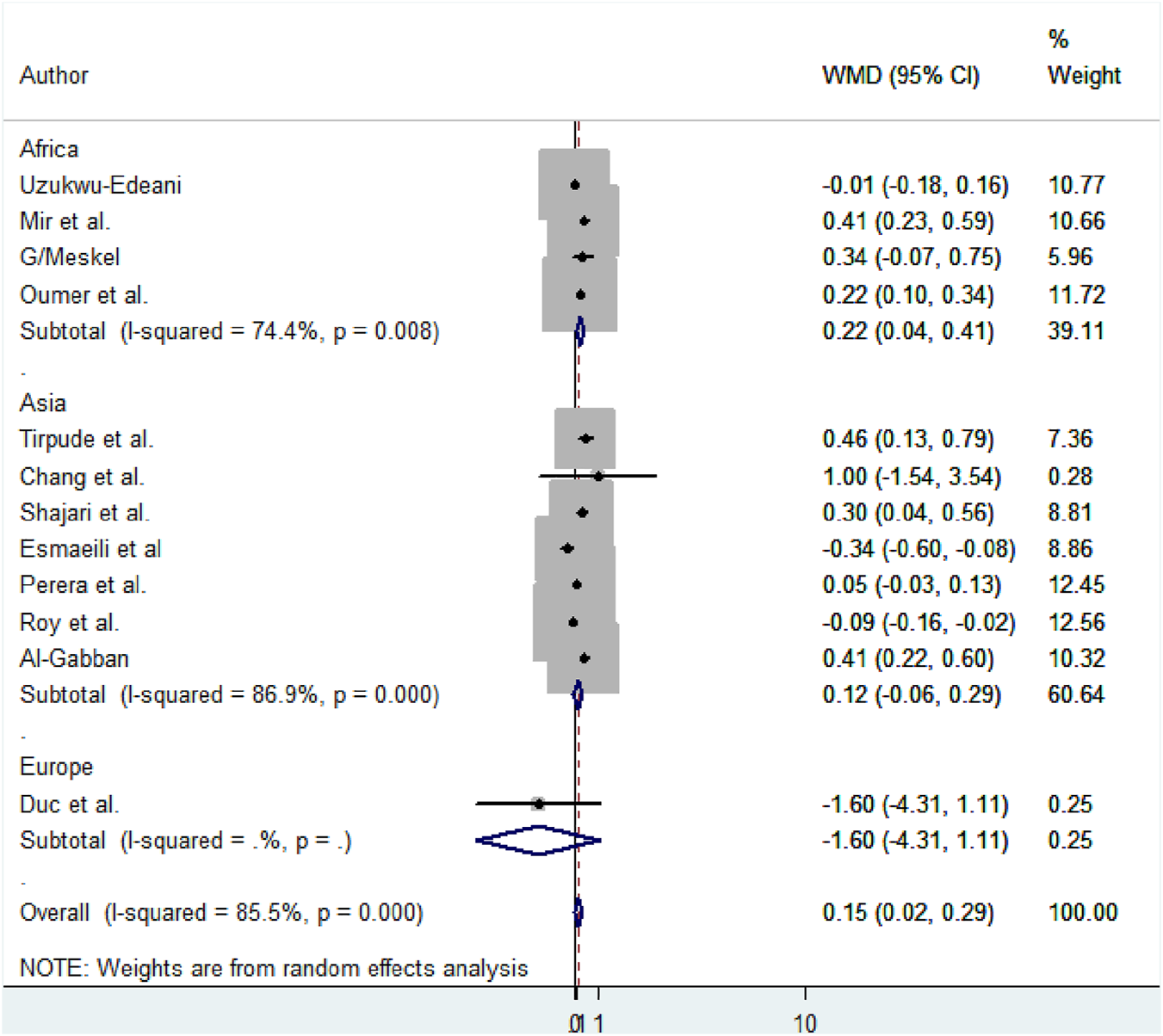
Subgroup analysis by region to show the variability of mean size difference of anterior fontanel among studies, 2020.
FIGURE 8
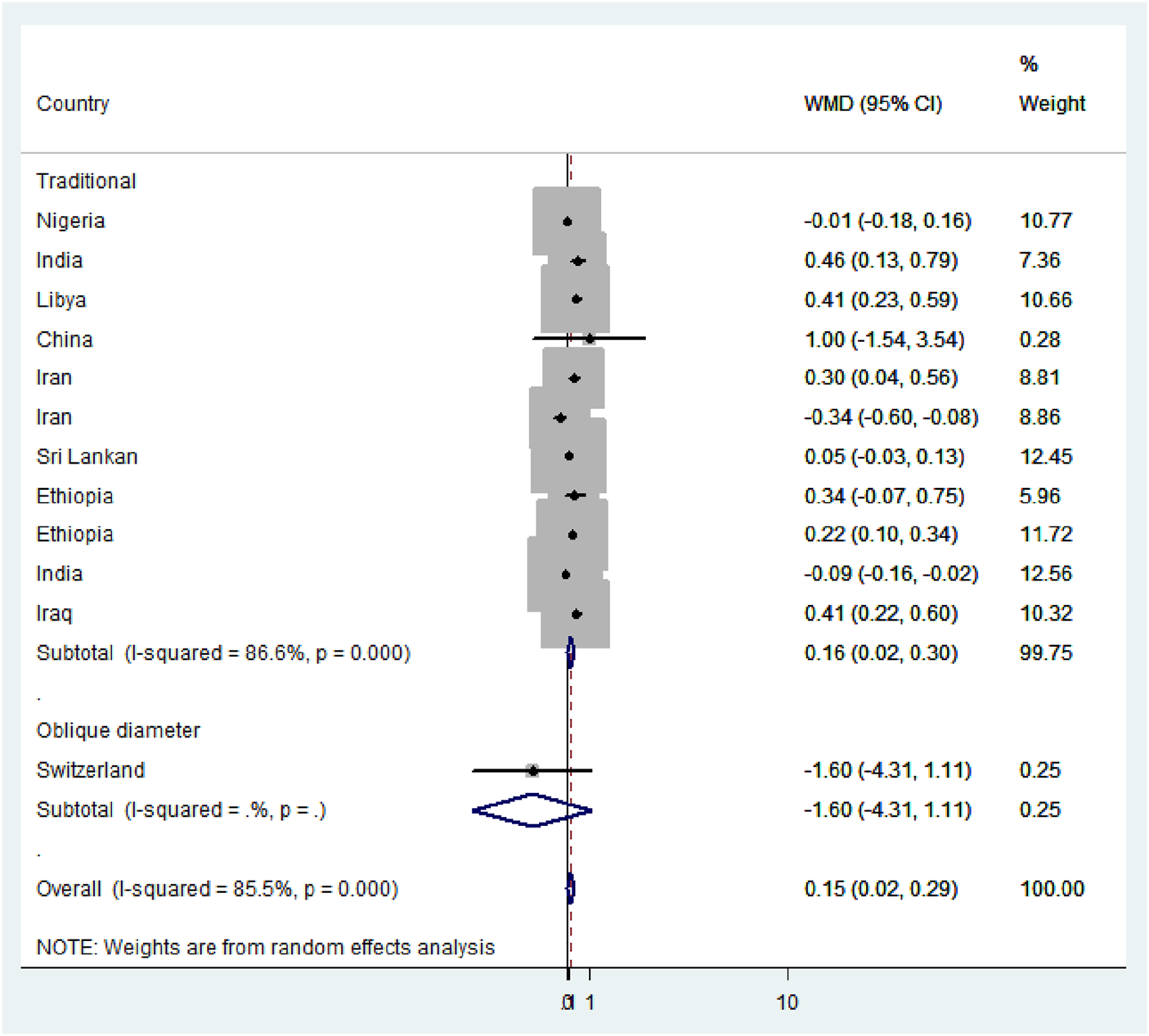
Subgroup analysis by methods, 2020.
FIGURE 9
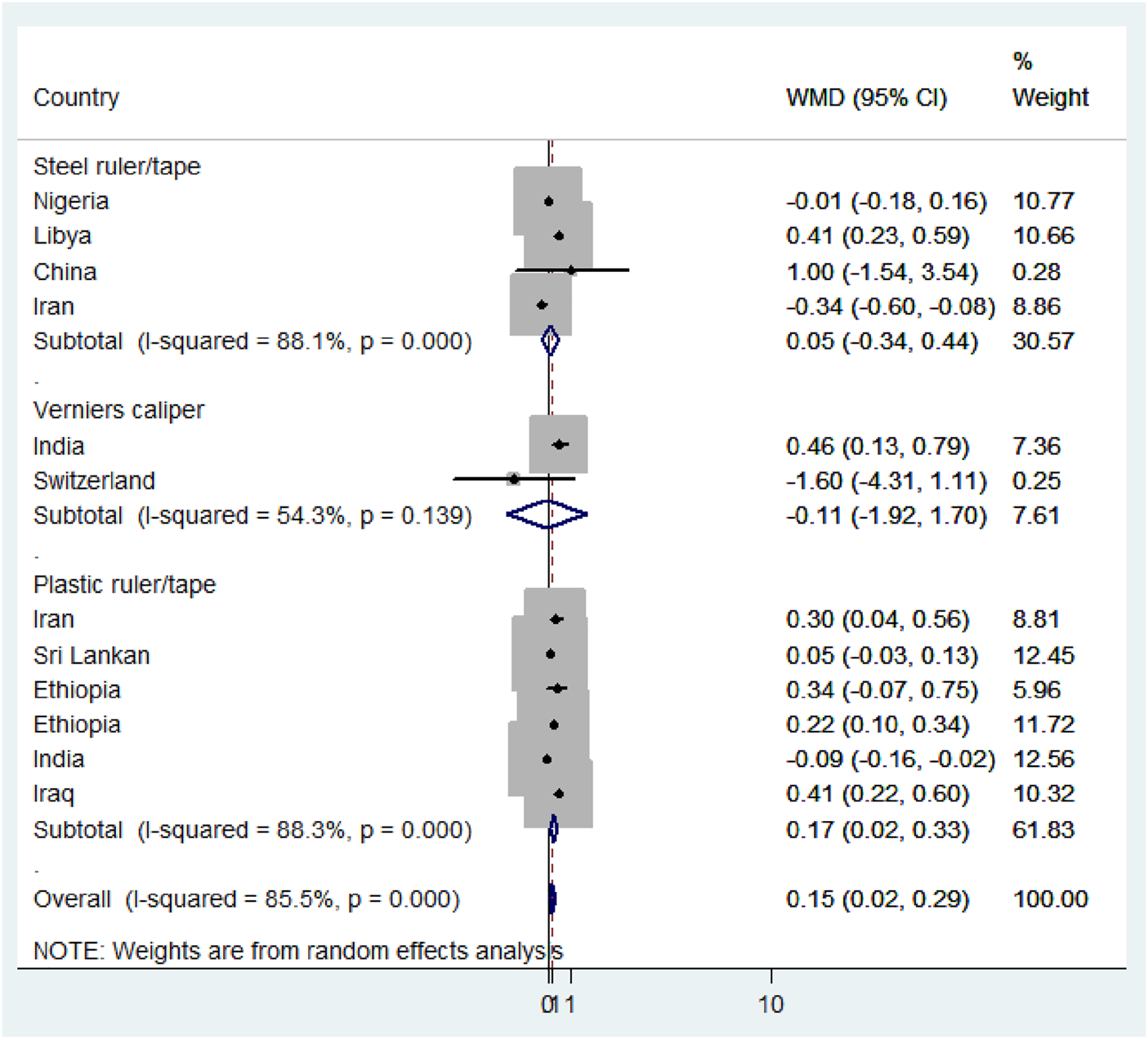
Subgroup analysis by measuring instruments, 2020.
In this meta-regression analysis, sample size (p-value = 0.62), study period (p-value = 0.77), quality score of studies (p-value = 0.65), year of publication (p-value = 0.91), study region/country (p-value = 0.95), study methods (p-value = 0.20), and measuring instruments (p-value = 0.26) were analyzed for the source of heterogeneity. None of them was statistically significant.
According to sensitivity analysis, the pooled estimation of this meta-analysis was not influenced by the studies. No individual study influences the overall estimate of the studies (Figure 10).
FIGURE 10
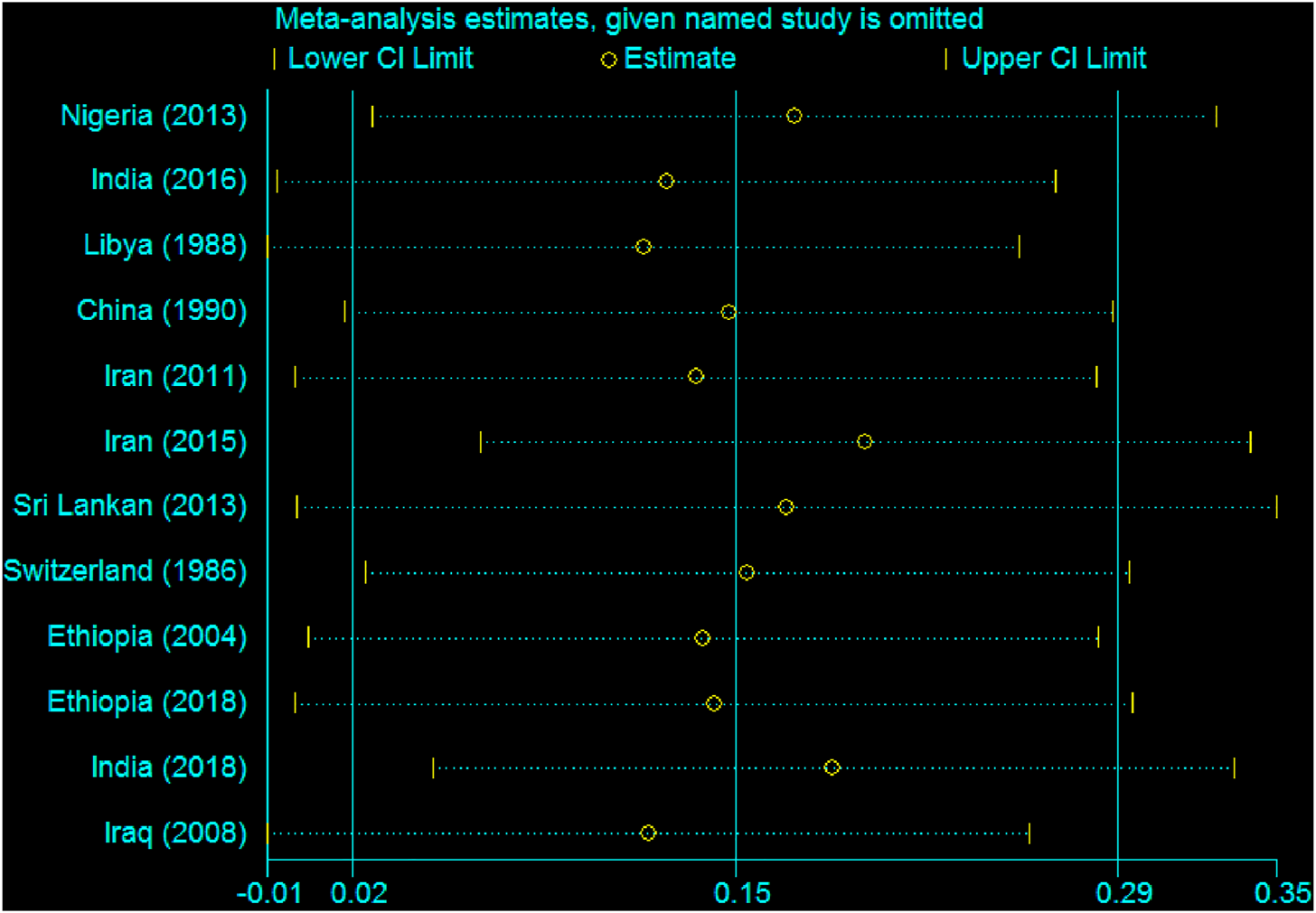
Sensitivity analysis showing the influence of individual studies, 2020.
Publication bias was also estimated using Egger’s regression tests (B-coefficient of bias = 1.60 (95% CI: 1.1, 4.3); p-value = 0.21) and Begg’s test (p-value = 0.95). Therefore, there was no statistically significant publication bias in estimating the pooled mean size of anterior fontanel. Besides, Egger’s publication bias plot supports the idea of non-significance (Figure 11).
FIGURE 11
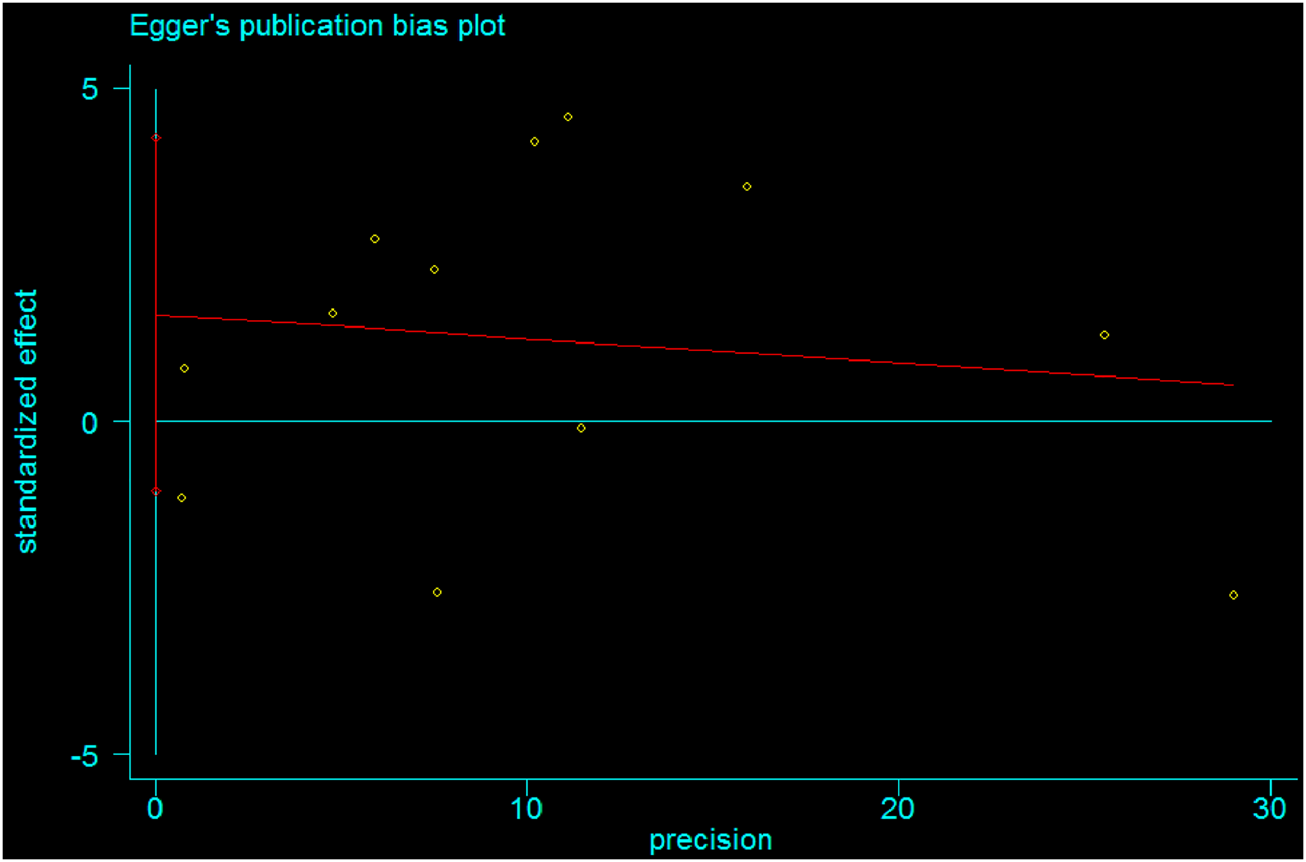
Egger's publication bias plot showing absence of bias among studies, 2020.
Discussion
This systematic review and meta-analysis were employed to determine the pooled mean size of anterior fontanel among term newborns globally based on available studies. Furthermore, it aimed to compare the mean size of the anterior fontanel between male and female newborns. There is a different view among studies regarding the size of anterior fontanel between male and female newborns. The evidence in this review provides the estimates of the pooled mean size of anterior fontanel globally, the overall mean difference between male and female newborns for anterior fontanel size, and the pooled mean size of anterior fontanel for different regions of the world. Interestingly, the findings of this study will have important implications for the clinical examination of the anterior fontanel size among newborns. Thus, it is important to understand the normal variations of anterior fontanel size in different regions and racial groups and the overall pooled reference value globally. Worldwide, everyday physicians carry out a physical examination on thousands of children. Physical examination of anterior fontanel size along with well-child care in neonates is highly recommended medical practices in Pediatrics [7, 8, 10]. It provides important evidence to follow the developmental status of the child and the general state of health. Besides, it can be considered as an index of cranial growth and development during the prenatal and postnatal periods. Any developmental alteration in anterior fontanel growth is an indicator of abnormal growth [7–9, 42].
In the present systematic review and meta-analysis, the pooled mean size of anterior fontanel was 2.58 cm. It can range between 2.31 and 2.85 cm. The heterogeneity across studies was assessed using the Cochrane Q test statistic, I2 test statistic, and p-values. A fixed-effect model was applied to estimate the pooled mean size of anterior fontanel due to the absence of heterogeneity. Graphically, the Forest and Galbraith’s plot visualized the absence of variability across the studies. In this meta-analysis, the pooled mean size of anterior fontanel for the Asia region was 2.49 cm, for the African region was 3.15 cm, for the America region was 2.35 cm, and for Europe region was 2.01 cm. The larger mean size was detected in the Africa region and a smaller mean size was found in Europe. The number of studies pooled was varied, seven in Africa, one in Europe, thirteen in Asia, and five in America. This review showed that there was no heterogeneity across study regions. Statistically, although there is no variation between the study regions or countries, the difference in the observed value was may be due to the difference in geography, genetics, or race. Besides, sample size and number of studies included in the pooled estimate may have also some influence. For instance, the number of included studies in Europe was only one.
The mean difference between male and female newborns was 0.15 cm. It can range between 0.02 and 0.29 cm. The random-effect model was applied to estimate the overall mean difference between male and female newborns. To deal with heterogeneity, sub-group analysis (based on region, method, measuring instruments, and study period, for example), meta-regression analysis, and sensitivity analysis were considered. The results of these subgroup analyses noted that the mean difference of anterior fontanel is significant among study regions, methods, and measuring instruments. Moreover, meta-regression analysis was performed based on the study region or country, methods, sample size, study period, the quality score of studies, year of publication, and measuring instruments. However, these included covariates in the meta-regression analysis were found not associated with the heterogeneity of the mean difference of anterior fontanel size between sexes. In this review, no study has a special influence over others on the overall estimation of meta-analysis. Essentially, all studies have uniform confidence intervals. A statistically significant mean difference was detected between males and females (MD = 0.15, 95% CI: 0.02, 0.29). The possible explanations for the observed differences in mean value between male and female newborns could be related to the differences in the study region or setting, race, genetics, nutrition, sample size, variation in measuring instruments, variation in methods used, and other methodological differences between the studies. The possible reason may be due to the fact that female newborns mostly had less body size as compared to male newborns (difference in birth weight, head circumference size, gestational age, and other anthropometric measurements).
Strength and Limitations of the Review
This review provided cumulative evidence in the estimation of anterior fontanel size, which is clinically very important. Besides, it gave a better understanding of anterior fontanel size and its mean difference between male and female newborns. As a limitation, some studies had not clear methodology (under-reported through publication) regarding the normal distribution of values to calculate the mean size of anterior fontanel. We considered only English written articles to meticulously evaluating the quality of the studies. Moreover, the gestational age of newborns (Even if we considered newborns appropriate for gestational age in our criteria, some studies did not explicitly state in the method part for the exclusion of small for gestational age and large for gestational age newborns in their study) and the adequacy of the sample size or variability in sample size may affect the estimated report. Furthermore, in this review, we did not analyze the correlation of anterior fontanel size with other anthropometric measurements at the time of birth (head circumference and birth weight, for instance) due to the inconsistency data related to standard deviations. Even if we performed subgroup analysis and meta-regression analysis for those mentioned covariates to minimize the variability or to identify the effect of covariates, we did not perform these analyses for the race, nutritional status, or antenatal care quantity and quality due to inadequacy of data in individual studies. Once more, this systematic review and meta-analysis considered that differences in strictly following the methods of measurement, such as measuring in the presence of wide sutures and inconsistent use of the same type of measuring instrument throughout the data collection period, may affect the pooled estimate of anterior fontanel size.
Conclusion
The pooled estimate of this review does provide the mean value of the anterior fontanel size in the newborns. There was a significant pooled mean fontanel size difference between male and female newborns. Thus, male newborns had a significantly larger mean fontanel size than female newborns.
Statements
Data availability statement
The data sets used and/or analyzed during the current systematic review and meta-analysis are available from the corresponding author on reasonable request.
Author contributions
MO, AT, and MA participated in conceptualization of the review protocol, formal analysis, methodology or study design, writing-original draft, interpretation, writing-review and editing, and approving the final draft. MO and MA: Quality assessment, data extraction, and literature review. All authors read and approved the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Kiesler J Ricer R . The Abnormal Fontanel. Am Fam Physician (2003). 67(12):2547–52.
2.
Moffett EA Aldridge K . Size of the Anterior Fontanelle: Three-Dimensional Measurement of a Key Trait in Human Evolution. Anat Rec (2014). 297:234–9. 10.1002/ar.22830
3.
Paladini D Vassallo M Sglavo G Pastore G Lapadula C Nappi C . Normal and Abnormal Development of the Fetal Anterior Fontanelle: a Three-Dimensional Ultrasound Study. Ultrasound Obstet Gynecol (2008). 32:755–61. 10.1002/uog.5368
4.
Crelin ES . Functional Anatomy of the Newborn. New Haven, CT: Yale University Press (1973)
5.
Nelson WE . Text Books of Pediatrics. 19th ed.USA: Elsevier-Saunders (2011)
6.
Aisenson M . Closing of the Anterior Fontanel. J Pediatr (1950). 6:223–6.
7.
Shajari H Rashidiranjbar N Ashrafy M . Anterior Fontanelle Size in Healthy Iranian Neonates on the First Day of Life. Acta Med Iran (2011). 49(8):543–6.
8.
Esmaeili M Esmaeili M Ghane Sharbaf F Bokharaie S . Fontanel Size from Birth to 24 Months of Age in Iranian Children. Iran J Child Neurol (2015). 9(4):15–23.
9.
G/Meskel T . The Size of Anterior Fontanel in Neonates and Infants in Addis Ababa. Ethiop Med J (2008). 46(1):47–53.
10.
Oumer M Guday E Teklu A Muche A . Anterior Fontanelle Size Among Term Neonates on the First Day of Life Born at University of Gondar Hospital, Northwest Ethiopia. PLoS ONE (2018). 13(10):e0202454. 10.1371/journal.pone.0202454
11.
Tirpude A Fulpatil M Kole S . Norms for Size and Closure Time of Anterior Fontanelle: a Study on Babies in Nagpur Region. Natl J Clin Anat (2016). 5(2):78–85. 10.4103/2277-4025.298191
12.
Popich GA Smith DW . Fontanels: Range of Normal Size. J Pediatr (1972). 80(5):749–52. 10.1016/s0022-3476(72)80125-2
13.
Uzukwu-Edeani CV Ibeziako SN Ikefuna AN Uchendu UO . Normal Anterior Fontanelle Sizes in Newborn Igbo Babies in South-Eastern Nigeria. S Afr J CH (2013). 7(2):50–3. 10.7196/sajch.516
14.
Mathur S Kumar R Mathur GP Singh VK Gupta V Tripathi VN . Anterior Fontanel Size. Indian Pediatr (1993). 31(2):161–4.
15.
Faix RG Durham NC . Fontanelle Size in Black and White Term Newborn Infants. J Pediatr (1982). 100(2):304–6. 10.1016/s0022-3476(82)80661-6
16.
Jackson G Hoyer A Longenecker L Engle W . Anterior Fontanel Size in Term and Late Preterm Hispanic Neonates: Description of Normative Values and an Alternative Measurement Method. Amer J Perinatol (2010). 27(4):307–12. 10.1055/s-0029-1241738
17.
Chakrabarti K . Anterior Fontanel Size in Hilly and Non-hilly Newborns in and Around the District of Darjeeling. Indian Pediatr (1989). 26:41–4.
18.
Duc G Largo RH . Anterior Fontanel: Size and Closure in Term and Preterm Infants. Pediatrics (1986). 78(5):904–8.
19.
Chang BF Hung KL . [Measurements of Anterior Fontanels in Chinese]. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi (1990). 31(5):307–12.
20.
Mir NA Weislaw R . Anterior Fontanelle Size in Arab Children: Standards for Appropriately Grown Full Term Neonates. Ann Trop Paediatrics (1988). 8(3):184–6. 10.1080/02724936.1988.11748566
21.
Perera P Wickramasinghe A Ranathunga N Fernanado M Warnakulasooriya D . Statistical Characteristics of Anterior Fontanelle Size at Birth of Term Sri Lankan New Borns: a Descriptive Cross Sectional Study. Ceylon Med J (2013). 58(3):96–100. 10.4038/cmj.v58i3.6102
22.
Lyall H Ogston SA Paterson CR . Anterior Fontanelle Size in Scottish Infants. Scott Med J (1991). 36(1):20–2. 10.1177/003693309103600111
23.
Roy S Vishnu VT Equba J . Anterior Fontanelle Size in Healthy Indian Late Preterm and Full Term Newborns. Indian J Pediatr (2018). 85(11):984-988. 10.1007/s12098-018-2690-4
24.
Algaban N . The Normal Standards of Anterior Fontanel Size in Iraqi Neonates. Iraqi J Comm Med (2008). 21(2):153–8.
25.
Omotade OO Kayode CM Adeyemo AA . Anterior Fontanelle Size in Nigerian Children. Ann Trop Paediatrics (1995). 15(1):89–91. 10.1080/02724936.1995.11747754
26.
Davies DP Ansari BM Cooke TJ . Anterior Fontanelle Size in the Neonate. Arch Dis Child (1975). 50:81–3. 10.1136/adc.50.1.81
27.
Moola S Munn Z Tufanaru C Aromataris E Sears K Sfetcu R et al Chapter 7: Systematic Reviews of Etiology and Risk. In: AromatarisEMunnZ, editors. Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute (2017) Available from: https://reviewersmanualjoannabriggsorg/
28.
Higgins JPT Thompson SG Deeks JJ Altman DG . Measuring Inconsistency in Meta-Analyses. Bmj (2003). 327(7414):557–60. 10.1136/bmj.327.7414.557
29.
Borenstein M Hedges LV Higgins JPT Rothstein HR . A Basic Introduction to Fixed-Effect and Random-Effects Models for Meta-Analysis. Res Synth Method (2010). 1(2):97–111. 10.1002/jrsm.12
30.
Egger M Smith GD Schneider M Minder C . Bias in Meta-Analysis Detected by a Simple, Graphical Test. Bmj (1997). 315(7109):6299310563–634. 10.1136/bmj.315.7109.629
31.
Begg CB Mazumdar M . Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics (1994). :1088–101.
32.
Moher D Liberati A Tetzlaff J Altman DG . The PRISMA Group: Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoSMed (2009). 6(7):e1000097. 10.1371/journal.pmed.1000097
33.
Ogunye O Ikeji MO Adeodu O . Craniofacial Dimensions in the African Neonate. Niger J Pediatr (1982) 9:21–5.
34.
El-Mougi M Zahran ME El-Sayed E El-Akkad N El-Sayed H El-Shafie E et al Age of Closure and Size of Anterior Fontanelle and its Relation to Anthropometric Measurements. Al-azhar Med J (1988). 17:75–9.
35.
Brandt I Hodes D Reimnitz P . Die große Fontanelle als Fenster zum Gehirn - Normalwerte und Verschlußzeiten. Klin Padiatr (1986). 198:330–6. 10.1055/s-2008-1033882
36.
Rizal AT Irawan M Aman BP . Head Circumference and Anterior Fontanel Measaurments in Newborns. Paediatr Indones (2012). 52(3):145–51. 10.14238/pi52.3.2012.145-51
37.
Adeyemo AA Olowu JA Omotade OO . Fontanelle Sizes in Term Neonates in Ibadan, Nigeria. West Afr J Med (1991). 18(1):55–9.
38.
Srugo I Berger A . Anterior Fontanelle Size in Healthy Israeli Newborn Infants. Isr J Med Sci (1987) 23(11):1137–9.
39.
Adeyemo AA Omotade OO . Variation in Fontanelle Size with Gestational Age. Early Hum Development (1999). 54:207–14. 10.1016/s0378-3782(98)00089-9
40.
Taksande A Jadhav A Biyani U . Measurment of Anterior Fontanel in Term Neonates in Rural Hospital of Central India (2015). 141–4.
41.
Tan K-L . Wide Sutures and Large Fontanels in the Newborn. Arch Pediatr Adolesc Med (1976) 130(4):386–90. 10.1001/archpedi.1976.02120050044007
42.
Malas MA Sulak O . Measurements of Anterior Fontanelle during the Fetal Period. J Obstet Gynaecol (2000). 20(6):601–5. 10.1080/01443610020001431
Summary
Keywords
anterior fontanel size, term newborn, mean difference, systematic review, meta-analysis
Citation
Oumer M, Tazebew A and Alemayehu M (2021) Anterior Fontanel Size Among Term Newborns: A Systematic Review and Meta-Analysis. Public Health Rev 42:1604044. doi: 10.3389/phrs.2021.1604044
Received
18 February 2021
Accepted
27 April 2021
Published
10 May 2021
Volume
42 - 2021
Edited by
Raquel Lucas, University Porto, Portugal
Updates
Copyright
© 2021 Oumer, Tazebew and Alemayehu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. PHR is edited by the Swiss School of Public Health (SSPH+) in a partnership with the Association of Schools of Public Health of the European Region (ASPHER)+
*Correspondence: Mohammed Oumer, mohammedoumer58@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.