- 1School of Public Health, University of Alberta, Edmonton, AB, Canada
- 2MAP Centre for Urban Health Solutions, Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, ON, Canada
- 3Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
- 4John W. Scott Health Sciences Library, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada
Objective: Recent evidence suggests that adequate fruit and vegetables intake (FVI) might be associated with lower risk of common mental disorders (CMDs) in adults, but studies in youth are also beginning to emerge and are synthesized in this systematic review.
Methods: Online databases were searched from inception to 30 October 2020 to locate cross-sectional, cohort, and case-control studies focusing on the FVI and CMDs in youth (i.e., 10–18 years old). The risk of bias of studies was assessed using Joanna Briggs Institute Critical Appraisal Tool and the Newcastle-Ottawa quality assessment scale.
Results: Among 3,944 records identified, 12 studies (8 cross-sectional, 1 case-control, and 3 prospective cohort studies) were included in the final synthesis. None of the prospective cohort studies identified a statistically significant association between FVI and CMDs in youth, although inconsistent associations were reported in cross-sectional and case-control studies.
Conclusion: The lack of associations between FVI and CMDs in youth, along with consistent associations in adults, might be explained by the accumulation of risk theoretical model and methodological challenges.
Introduction
Mental disorders constitute a significant global public health burden [1–3] and result from an intricate interplay of multiple factors. This interplay is particularly potent during adolescence (i.e., 10–19 years old) when more than three-quarters of all life-time mental disorders, especially common mental disorders (CMDs) such as depression and anxiety, manifest for the first time [4, 5]. In fact, the cumulative probability of CMDs rises steeply from around 5% in early adolescence to as high as 20% by the end of adolescence [6]. Mental disorders in adolescence are associated with many long-term negative psychosocial outcomes, including low educational attainment, poor work performance [7], difficulty developing stable relationships and social networks, unemployment [8], as well as future mental disorders [9], substance abuse, and suicide [6]. Often underdiagnosed [4], they can be difficult to manage due to limited effectiveness of available treatment options [10] and high rates of recurrence [6] and comorbidity [11].
With one in five adolescents living with mental disorders worldwide [12], there is a recognized need for the development and implementation of effective primary prevention strategies [13]. Recent systematic reviews [14, 15] of observational studies point to the association between adherence to high quality diet (i.e., rich in fruit, vegetables, legumes, nuts and whole grains [16]) and lower incidence of CMDs in a general population of youth. However, diet quality is often conceptualized and measured differently, making it difficult to compare results across different studies. To circumvent this challenge, fruit and vegetables intake (FVI) is often used as a simple indicator of overall diet quality [17].
A systematic review [18] of 16 cross-sectional, 9 cohort, and 2 case-control on the association between FVI and mental disorders in adults showed that the highest category of FVI was associated with up to 17% lower risk of depression in cohort studies, with higher magnitudes of the associations (i.e., up to 25% lower risk of depression) observed in cross-sectional studies. Moreover, every 100-g increase in fruit and vegetable intake was associated with a 3% reduced risk of depression in cohort studies. However, to our knowledge, evidence on this association in adolescents has not yet been synthesized. This paper fills in this gap, with particular attention given to the methodological aspects of available studies to inform future research in the field of nutritional psychiatry in youth.
Methods
Search Strategy
A medical librarian [SC (Sandra Campbell)] searched the following databases: Prospero, Wiley Cochrane Library, Ovid Embase, Ovid Medline, Ovid PsycInfo, EBSCO CINAHL, ProQuest Dissertations and Theses Global, Food Science and Technology Abstracts (WOS) and CAB Abstracts (WOS). Each of the databases was searched from inception to 30 October 2020. The search strategy included both text words and controlled vocabulary (e.g., MeSH, EMTREE, etc.) for the terms “fruits or vegetables” and “anxiety or depression” (see Supplementary File S1). Studies limited to adults and very young children were excluded. In addition, bibliographies of relevant studies and researcher-identified databases were hand searched. All identified records were exported to Covidence systematic review software [19], and duplicates were automatically removed (see PRISMA flowchart, Supplementary File S2). Language restriction was not applied; when necessary, a native language speaker was identified in the research community at the University of Alberta and asked to translate the paper, assess eligibility of the study and extract data. This systematic review was registered on PROSPERO (CRD42020148625, 1 August 2020).
Inclusion Criteria
Two reviewers [JD and SM] independently reviewed the titles and abstracts on the Covidence platform [19]. [JD and SM] documented and compared reasons for exclusion. Bibliographies of included papers were reviewed for relevant papers independently by [JD and SM]. Disagreements during the screening process were resolved by consensus. We included observational (i.e., cohort, case-control, and cross-sectional) studies that focused on FVI, measured combined or separately, and CMDs in community-dwelling adolescents (see detailed inclusion and exclusion criteria in Table 1). Inclusion of primary studies was not limited by sex, ethnicity, or any socioeconomic determinants of health. Studies that included adolescents but also extended outside the specified age range (i.e., 10–18 years old) were assessed on a case-by-case basis as to whether they could meaningfully contribute to the systematic review. If data were duplicated in more than one study, only the latest study or the study with the largest sample size was considered. Studies that did not report any estimates of the association of interest and did not provide any data that could be used to calculate any measures of association were excluded.
Data Extraction and Management
Upon finalizing the list of included studies, [JD and SM] independently extracted the following data: study details (first author, title, publication year, journal, objectives of the study, study design, follow-up years, study duration, recruitment procedures utilized, description of the exposure(s) and exposure assessment tools, comparator, description of the outcome(s) and outcome assessment tools, sample size, mean age or age range at baseline, sex of participants included in the study); analysis and results (i.e., statistical methods used to produce the measure and magnitude of association, standard error, standard deviation for the exposure and control groups, 95% CI, p-value, confounders adjusted for in the analysis); and author conclusions. If data in a selected study was missing or lacked sufficient details, [JD] contacted corresponding authors for additional information. Where the results of several models where presented, data were extracted for all models. Study-specific methods and results (e.g., statistical methods used, comparators, effect measures) are presented in Supplementary Table S1 (Supplementary File S3).
Risk of Bias Assessment
The Newcastle-Ottawa quality assessment scale [20] was used to assess the quality of cohort and case-control studies. The Joanna Briggs Institute (JBI) Critical Appraisal Tool for cross-sectional studies [21] was used to assess the quality of cross-sectional studies. One question was excluded from this tool (i.e., “Were objective, standard criteria used for measurement of the condition?”), since the focus was on the general population rather than specific diagnostic methods or clinical populations. As part of the quality assessment process, we assessed whether important confounding factors [particularly, socioeconomic status (SES) which can affect both person’s diet and mental health outcomes] were identified and adjusted for in the included studies. Discrepancies resulting from the independent application of quality assessment tools by [JD and SM] were resolved by consensus. Since the systematic review aimed to map the existing literature and highlight methodological challenges and areas for further research, studies were not excluded based on risk of bias assessment. The utility of introducing a qualitative score has long been discredited [33]; instead, quality assessments of the selected cross-sectional, cohort and case-control studies are summarized in Tables 2–4, respectively.

TABLE 2. Quality assessment of cross-sectional studies included in the systematic review (systematic review, all countries, up to 2020).
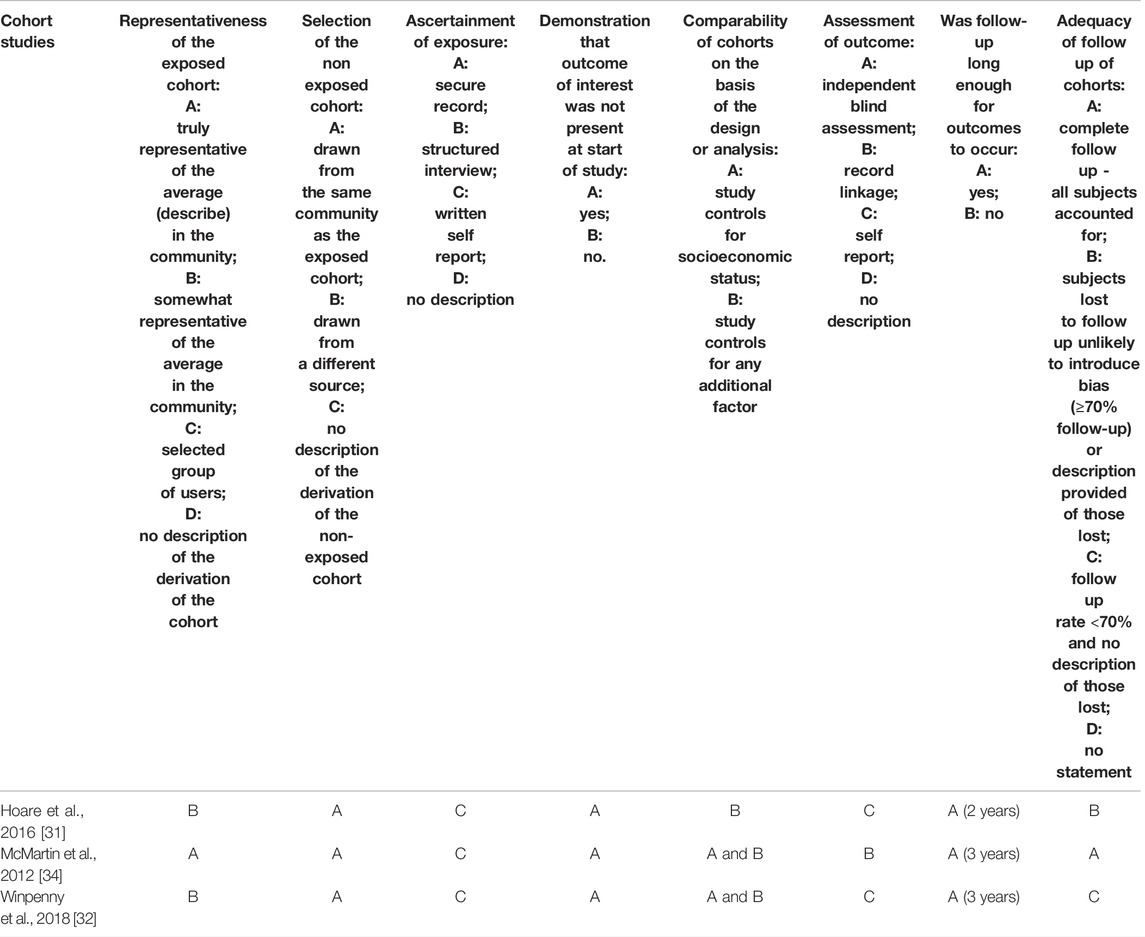
TABLE 3. Quality assessment of cohort studies included in the systematic review (systematic review, all countries, up to 2020).

TABLE 4. Quality assessment of case-control studies included in the systematic review (systematic review, all countries, up to 2020).
Data Analysis and Reporting
Considering substantial heterogeneity between studies in terms of study design, exposure and outcome definition and assessment methods, included covariates, measures of association, and results of the risk of bias assessment, the authors refrained from pooling data in a meta-analysis. Moreover, the number of studies available was not sufficient to pool in sub-group analyses. Therefore, a narrative synthesis is provided. The review follows Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines (Supplementary File S4).
Results
The search yielded 3,944 records to assess for eligibility, 903 duplicates were removed. Among 117 records that were examined in full, 12 studies were included in the final analysis. The articles were published between 2012 and 2020 and included analysis of data collected in United States, United Kingdom, Greece, Australia, Canada, South Korea, Saint Lucia, Egypt, Saint Vincent and the Grenadines, Djibouti, Morocco, Myanmar, Zambia, Tanzania, Venezuela, Grenada, Lebanon, China, Indonesia, Thailand, Uganda, Tunisia, Botswana, Sri Lanka, India, Seychelles, Guyana, Ecuador, Jordan, Argentina, and Kenya. Table 5 and Supplementary Table S1 (see Supplementary File S4) provide detailed information on the study characteristics and results of the included studies, respectively. Eight studies [23–27, 28–30] used cross-sectional design, three studies [31, 32, 34] were prospective cohort studies (two [31, 32] included analyses of both cross-sectional and longitudinal data), and one [35] was a case-control study. Sample sizes ranged from 603 [32] to 65,528 [28] adolescents in cross-sectional studies, from 472 [31] to 3,757 [34] in cohort studies, and 849 [35] in the case-control study. Except for one study that focused exclusively on female youth [35], all other studies included approximately equal numbers of male and female youth. Age distribution was within the prespecified age limits (i.e., 10–18 years old) in all but one study [25] that included a range of 9–13 years old children attending Grade 5 and 6, with 58% of the sample being older children (11–13 years old).
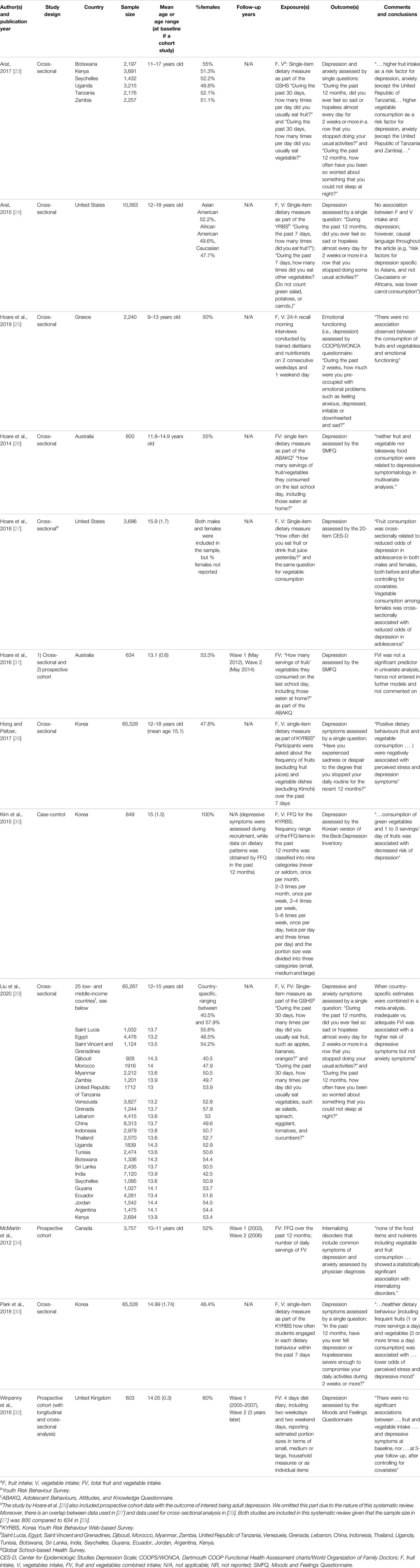
TABLE 5. Description of studies included in the systematic review (systematic review, all countries, up to 2020).
Only self-reported dietary assessment instruments were used. Eight studies [23–25, 27–30, 35] assessed intakes of fruit and vegetables separately, while five studies [26, 29, 31, 32, 34] assessed combined FVI. Seven studies [23, 24, 27–30, 35] measured FVI in terms of frequency of FV consumption, while five studies [25, 26, 31, 32, 34] assessed FVI in terms of servings or grams per day. Food frequency questionnaire [34], four-day diet diary [32], and 24-h dietary recall [25] were used in one study each. Single-item dietary assessment questions were part of larger questionnaires on various lifestyle behaviours in nine studies [23, 24, 26–31, 35]. The questions referred to fruit, vegetable, or combined intake in the past 12 months [34, 35], 30 days [23, 29], 7 days [24, 28, 30], and the day before [26, 27, 31].
All studies assessed depression, two assessed anxiety [23, 29], and one study [34] included common symptoms of depression and anxiety (combined) as the outcome of interest. Six studies used the following validated questionnaires to assess the outcomes of interest: COOPS/WONCA questionnaire [25], SMFQ [26, 31], CES-D [27], Korean version of the Beck Depression Inventory [35], and Moods and Feelings Questionnaire [32]. One study [34] included physician diagnoses of internalizing disorders, and the rest of the studies [23, 24, 28–30] used single-item self-reported questions to assess the outcome(s) of interest. Subgroup analysis based on gender was conducted in five studies [25–27, 31, 32].
Quality Assessment
Of 10 cross-sectional studies, eight [23–28, 31, 32] omitted the criteria for inclusion in the sample, and five [23, 24, 28–30] did not include validated scales to assess CMDs. Causal claims in respect to the focal association were made in two [23, 24] cross-sectional studies. All (except one [24]) studies identified and adjusted for at least some important confounding factors: four studies [25, 27, 28, 30] included indicators of SES and one [23] adjusted for food insecurity, two studies stratified by ethnicity [24] and gender [31].
There was one [35] case-control study. Community controls with no history of disease were selected from the same source population as the cases (i.e., adolescent youth attending the University Health Center for annual routine health examinations). FFQ with 63 food items and the Korean version of the Beck Depression Inventory (K-BDI) were included in the same questionnaire. Importantly, participants with pre-existing psychological conditions or those taking medication for depression were excluded from the study. Some of the most important confounding factors, such as SES, familial history of depression and physical activity, were not measured. It is unclear whether assessors were blinded to the research question or outcome assessment.
Among three prospective cohort studies, samples were representative of 10–11 years old children in corresponding communities in one study [34]. In all cohort studies, non-exposed cohorts were drawn from the same community as exposed cohorts and samples appeared free from mental disorders at the beginning of the studies. All studies ascertained exposure using self-reported dietary measures, and in all but one [34] mental disorders were self-reported. One study [31] included a two-year follow-up, while the other two studies included three-year [32, 34] follow-ups. One study [34] had complete follow up. Another study [31] had 74.5% participation rate, but participants lost to follow-up (3.2% refusal, 7.2% unavailable, 15.1% relocated) were unlikely to introduce bias. One study [32] reported a follow-up rate of less than 70%; the analysis of differences between participants and non-participants was not available, making it difficult to assess the possibility of selection bias. All studies adjusted for sex/gender. Other confounding factors adjusted for included: parental education, and the school participants attended [31]; age, SES, other lifestyle behaviours (smoking, physical activity, alcohol consumption, sleep), friendship quality, self-esteem, family functioning, %body fat, medication use, total energy intake, and depression symptoms at baseline [32]; and household income, parental marital status, parental education, body weight status, physical activity and geographic area [34].
F&V Intakes (Measured Separately) and Depression
Eight studies (seven cross-sectional [23–25, 27–30] and one case-control [35]) reported inconsistent associations between fruit and vegetable intakes and depression symptoms. For example, the study by Liu et al. [29] that analyzed the association between depression and fruit and vegetable intakes in 25 low- and middle-income countries reported statistically significant associations. While the associations between fruit intake and depression were statistically significant for some countries (e.g., Tanzania, China, Indonesia, Thailand, India, Seychelles, Ecuador, and Jordan), they were not statistically significant for other low- and middle-income countries (Saint Lucia, Egypt, Saint Vincent and the Grenadines, Djibouti, Morocco, Myanmar, Zambia, Venezuela, Grenada, Lebanon, Uganda, Tunisia, Botswana, Sri Lanka, Guyana, Argentina, and Kenya). Associations in all listed countries were adjusted for different potential confounding factors. Liu et al. pooled data from all studies in a meta-analysis: fruit intake of <2 times and 2 or more times/day versus none was associated with 0.79 (0.73; 0.86) and 0.75 (0.68, 0.82) times lower odds of depression, respectively. Vegetable intake of <3 times/day and 3 or more times per day vs. none was associated with 0.74 (0.67; 0.83) and 0.75 (0.68; 0.84) times lower odds of depression, respectively.
FVI (Combined) and Depression
Four studies reported on the association between combined FVI and depression. Two studies were cross-sectional, while two others included both cross-sectional and longitudinal analyses. One cross-sectional study [29] reported statistically significant associations in four of 25 low- and middle-income countries (i.e., Seychelles, Ecuador, Jordan, Kenya). One prospective study reported non-significant associations in univariate analyses: OR = 0.87 (95% CI 0.39; 1.96) for males and OR = 0.85 (95% CI 0.54; 1.34) for females [31]. Another prospective cohort study reported no statistically significant associations following adjustment for covariates: ß = 0.14 (95% CI of −0.15; 0.43) [32].
F&V Intakes (Measured Separately) and Anxiety
Two studies [23, 29] examined the associations between fruit and vegetable intakes and anxiety. Arat [23] reported results for six of the low- and middle-income countries and found statistically significant associations in Botswana, Kenya (for fruit but not vegetable intake as the exposure of interest), Seychelles, Uganda, Tanzania, and Zambia. Another study by Liu et al. [29] used data from the same questionnaire as Arat [23], although for a narrower age range, reporting statistically significant associations between fruit intake and anxiety for Morocco, Tanzania, Venezuela China, Indonesia, Uganda, Tunisia, Sri Lanka, India, Ecuador, Jordan, Argentina, Kenya; and between vegetable intake and anxiety in Saint Vincent and the Grenadines, Djibouti, Lebanon, China, Seychelles, and Ecuador. When the measures of association were combined in a meta-analysis, the fruit intake of <2 times/day and 2 or more times a day compared to no intake was associated with 0.60 (0.54; 0.67) and 0.61 (0.54, 0.68) times lower odds of anxiety, while vegetable intake of <3 times/day and 3 or more times a day versus no intake was associated with 0.71 (0.63; 0.81) and 0.87 (0.73; 1.03) times lower odds of having anxiety symptoms. Neither of the studies reported on the association between the combined FVI and anxiety.
FVI (Combined) and Depression and Anxiety (Combined)
One study [34] concluded that there was no statistically significant association between FVI and internalizing disorders when comparing second tertile to first tertile (IRR 1.04, 95% CI 0.71; 1.53) and third tertile to first tertile (IRR 1.25, 95% CI 0.8; 1.99). Analyses were adjusted for energy intake, gender, household income, parental marital status and education, body weight status, physical activity, and geographical area.
Discussion
Fruit and vegetables have long been recognized for their beneficial effects on gastrointestinal health, weight management, prevention of cardiovascular and metabolic disorders, respiratory health, and high bone mineral density, among other conditions and diseases [36]. Moreover, FVI has recently been shown to be associated with lower risk of mental disorders in the general population [18, 37]. However, our systematic review did not confirm previous claims for the existing association between FVI and CMDs specifically in youth. Among 12 identified studies, one case-control and some of the cross-sectional studies pointed to significant associations between FVI and CMDs in youth, while none of the three prospective cohort studies showed significant associations after adjusting for confounding factors.
Previously proposed biological mechanisms to explain the association between FVI and CMDs revolve around the high content of fiber, nutrients (e.g., vitamin C), and phytochemicals (e.g., polyphenols, carotenoids) [36] found in vegetables and fruits, which are believed to have beneficial effects on neurotransmitter systems, neuronal plasticity [38], and gut health [39–41]. Although the aforementioned biological mechanisms appear plausible and are supported by studies in adults, the effects of FVI on CMDs may differ in youth due to the rapid brain development during adolescence [42]. Another potential explanation involves one of the existing theoretical models derived from life course epidemiology—i.e., the accumulation of risk model [43]. This model states that every additional year of exposure is associated with an increased risk of poor outcomes: this could explain why the association between FVI and CMDs becomes apparent later in life. Further research looking at the diet-mental health relationship through the lens of life course epidemiology is warranted. Methodological challenges, discussed below, could also explain our findings.
Consistent with other literature on the diet-mental health relationship [15, 18, 44], cross-sectional study design was the one most commonly used. While the cross-sectional study design can help generate hypotheses, in respect to the diet-mental health relationship this task has already been fulfilled. The inability to determine the temporal order of diet and mental disorders makes this study design of limited value to any etiological inferences [45]. Moreover, cross-sectional studies identified in this systematic review provided inconsistent conclusions, potentially due to adjusting for different confounding factors. As for the case-control study [35], the authors excluded those with pre-existing mental disorders, thus partly tackling the issue of reverse causality, but we cannot exclude the possibility of recall bias. Both exposure and outcome were assessed at the same time, and the study did not report whether those who completed the dietary assessment were blinded to participants’ outcomes or the research question itself. Given the aforementioned limitations inherent to cross-sectional and case-control study designs and in the absence of prevention trials (in part due to ethical and feasibility concerns), attention and efforts should be redirected to planning and conducting rigorous prospective cohort studies. We identified three prospective cohort studies and, given the incremental nature of research, more prospective cohort studies that address the methodological issues outlined below would be of value.
First of all, SES is an established confounder linked to both diet and mental disorders and therefore should be controlled for in all studies investigating this focal relationship; SES was measured and adjusted for in two [32, 34] out of three cohort studies included in this systematic review. At the same time, some of the variables (e.g., weight status indicators) that were treated as confounding factors could well be intermediate variables that we should not control for. Additionally, it is important to consider the nature of confounding factors (e.g., time-invariant such as ethnicity, race, sex vs. time-variant such as food security, parental mental health, family functioning), which could inform the choice of analytical methods (e.g., parametric G-formula) other than the standard regression models (e.g., linear and logistic regression models). Employing directed acyclic graphs [46] could help guide these pre-analysis steps and identify appropriate adjustment sets, minimize inappropriate adjustment, and invite external scrutiny to enhance the quality of work.
In addition, there are measurement errors associated with both self-reported diet and mental disorders. Given the potential for recall and social desirability biases associated with self-report measures, sensitivity analyses to delineate the effects of measurement errors on the focal relationship are needed [47]. Adjustment for total energy intake is another strategy that has been strongly recommended to partially correct for the measurement error associated with self-reported dietary intake. Moreover, fruit juice is excluded from recent healthy eating recommendations due to excess free sugars they contain [48]; for this reason, consumption of fruit juice should not count toward FVI. In addition, validated questionnaires, as opposed to single-item screener questions, should be preferred for the assessment of mental health disorders. Lastly, despite pronounced sex differences in both the prevalence of mental disorders [49] and eating behaviours and diet [50, 51], sub-group analysis was done in less than half of the included studies, and further exploration of potential effect modification is of value.
Conclusion
This systematic review showed that while inconsistent associations between FVI and CMDs in youth were reported in cross-sectional and case-control studies, no association was detected in prospective cohort studies. This evidence differs from what has recently been concluded in a systematic review on the association between FVI and depression in adults [18], which can be explained by the accumulation of risk theoretical model of the development of mental disorders and/or methodological challenges outlined in the paper.
Author Contributions
JD conceptualized the idea for the systematic review and is the guarantor of the review. SC executed searches in all databases. SM was the second reviewer. All authors contributed to the initial draft and reviewed the subsequent drafts and the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/phrs.2022.1604686/full#supplementary-material
References
1.National Institute of Mental Health. Mental Illness Definitions (2022). Available from: https://www.nimh.nih.gov/health/statistics/mental-illness.shtml (Accessed August 8, 2022).
2. Vigo, D, Thornicroft, G, and Atun, R. Estimating the True Global burden of Mental Illness. Lancet Psychiatry (2016) 3(2):171–8. doi:10.1016/S2215-0366(15)00505-2
3.World Health Organization. Global burden of Mental Disorders and the Need for a Comprehensive, Coordinated Response from Health and Social Sectors at the Country Level (2012). Available from: https://apps.who.int/iris/bitstream/handle/10665/78898/A65_10-en.pdf?sequence=1&isAllowed=y (Accessed October 9, 2019).
4. Kessler, RC, Berglund, P, Demler, O, Jin, R, Merikangas, KR, and Walters, EE. Lifetime Prevalence and Age-Of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry (2005) 62(6):593–602. doi:10.1001/archpsyc.62.6.593
5. Merikangas, KR, He, JP, Burstein, M, Swanson, SA, Avenevoli, S, Cui, L, et al. Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry (2010) 49(10):980–9. doi:10.1016/j.jaac.2010.05.017
6. Thapar, A, Collishaw, S, Pine, DS, and Thapar, AK. Depression in Adolescence. Lancet (2012) 379(9820):1056–67. doi:10.1016/S0140-6736(11)60871-4
7. Johnson, D, Dupuis, G, Piche, J, Clayborne, Z, and Colman, I. Adult Mental Health Outcomes of Adolescent Depression: a Systematic Review. Depress Anxiety (2018) 35(8):700–16. doi:10.1002/da.22777
8. Clayborne, ZM, Varin, M, and Colman, I. Systematic Review and Meta-Analysis: Adolescent Depression and Long-Term Psychosocial Outcomes. J Am Acad Child Adolesc Psychiatry (2019) 58(1):72–9. doi:10.1016/j.jaac.2018.07.896
9. Colman, I, Naicker, K, Zeng, Y, Ataullahjan, A, Senthilselvan, A, and Patten, SB. Predictors of Long-Term Prognosis of Depression. CMAJ (2011) 183(17):1969–76. doi:10.1503/cmaj.110676
10. Cipriani, A, Zhou, X, Del Giovane, C, Hetrick, SE, Qin, B, Whittington, C, et al. Comparative Efficacy and Tolerability of Antidepressants for Major Depressive Disorder in Children and Adolescents: a Network Meta-Analysis. Lancet (2016) 388(10047):881–90. doi:10.1016/S0140-6736(16)30385-3
11. Angold, A, and Costello, EJ. Depressive Comorbidity in Children and Adolescents: Empirical, Theoretical, and Methodological Issues. Am J Psychiatry (1993) 150(12):1779–91. doi:10.1176/ajp.150.12.1779
12. Kessler, RC, and Bromet, EJ. The Epidemiology of Depression across Cultures. Annu Rev Public Health (2013) 34:119–38. doi:10.1146/annurev-publhealth-031912-114409
13. McDaid, D, Park, AL, and Wahlbeck, K. The Economic Case for the Prevention of Mental Illness. Annu Rev Public Health (2019) 40(1):373–89. doi:10.1146/annurev-publhealth-040617-013629
14. O’Neil, A, Quirk, SE, Housden, S, Brennan, SL, Williams, LJ, Pasco, JA, et al. Relationship between Diet and Mental Health in Children and Adolescents: a Systematic Review. Am J Public Health (2014) 104(10):e31–42. doi:10.2105/AJPH.2014.302110
15. Khalid, S, Williams, CM, and Reynolds, SA. Is There an Association between Diet and Depression in Children and Adolescents? A Systematic Review. Br J Nutr (2016) 116(12):2097–108. doi:10.1017/S0007114516004359
16.World Health Organization. Healthy Diet (2020). Available from: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (Accessed September 20, 2021).
18. Saghafian, F, Malmir, H, Saneei, P, Milajerdi, A, Larijani, B, and Esmaillzadeh, A. Fruit and Vegetable Consumption and Risk of Depression: Accumulative Evidence from an Updated Systematic Review and Meta-Analysis of Epidemiological Studies. Br J Nutr (2018) 119(10):1087–101. doi:10.1017/S0007114518000697
19.Veritas Health Innovation. Covidence Systematic Review Software. Melbourne, Australia: Veritas Health Innovation (2020).
20. Wells, GA, Shea, B, O’Connell, D, Peterson, J, Welch, V, Losos, M, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐analyses (2008). Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed August 15, 2019).
21.JBI Reviewer’s Manual. Appendix 7.5 Critical Appraisal Checklist for Analytical Cross-Sectional Studies (2019). Available from: https://wiki.joannabriggs.org/display/MANUAL/ Appendix+7.5+Critical+appraisal+checklist+for+ analytical+cross-sectional+studies (Accessed August 9, 2019).
22.World Health Organization. Fruit and Vegetables for Health. Report of a Joint FAO/WHO Workshop. Kobe, Japan: World Health Organization (2004).
23. Arat, G. The Link between Nutrition and Mental Health in Sub-saharan African Adolescents: Findings from the Global School-Based Health Survey. Glob Soc Welf (2017) 4(1):31–40. doi:10.1007/s40609-016-0046-4
24. Arat, G. Emerging Protective and Risk Factors of Mental Health in Asian American Students: Findings from the 2013 Youth Risk Behavior Survey. Vulnerable Child Youth Stud (2015) 10(3):192–205. doi:10.1080/17450128.2015.1045437
25. Hoare, E, Marx, W, Firth, J, McLeod, S, Jacka, F, Chrousos, GP, et al. Lifestyle Behavioural Risk Factors and Emotional Functioning Among Schoolchildren: The Healthy Growth Study. Eur Psychiatry (2019) 61:79–84. doi:10.1016/j.eurpsy.2019.07.002
26. Hoare, E, Millar, L, Fuller-Tyszkiewicz, M, Skouteris, H, Nichols, M, Jacka, F, et al. Associations between Obesogenic Risk and Depressive Symptomatology in Australian Adolescents: a Cross-Sectional Study. J Epidemiol Community Health (2014) 68(8):767–72. doi:10.1136/jech-2013-203562
27. Hoare, E, Hockey, M, Ruusunen, A, and Jacka, FN. Does Fruit and Vegetable Consumption during Adolescence Predict Adult Depression? A Longitudinal Study of US Adolescents. Front Psychiatry (2018) 9:581. doi:10.3389/fpsyt.2018.00581
28. Hong, SA, and Peltzer, K. Dietary Behaviour, Psychological Well-Being and Mental Distress Among Adolescents in Korea. Child Adolesc Psychiatry Ment Health (2017) 11:56. doi:10.1186/s13034-017-0194-z
29. Liu, MW, Chen, QT, Towne, SD, Zhang, J, Yu, HJ, Tang, R, et al. Fruit and Vegetable Intake in Relation to Depressive and Anxiety Symptoms Among Adolescents in 25 Low- and Middle-Income Countries. J Affect Disord (2020) 261:172–80. doi:10.1016/j.jad.2019.10.007
30. Park, S, Rim, SJ, and Lee, JH. Associations between Dietary Behaviours and Perceived Physical and Mental Health Status Among Korean Adolescents. Nutr Diet (2018) 75(5):488–93. doi:10.1111/1747-0080.12444
31. Hoare, E, Millar, L, Fuller-Tyszkiewicz, M, Skouteris, H, Nichols, M, Malakellis, M, et al. Depressive Symptomatology, Weight Status and Obesogenic Risk Among Australian Adolescents: a Prospective Cohort Study. BMJ Open (2016) 6(3):e010072. doi:10.1136/bmjopen-2015-010072
32. Winpenny, EM, van Harmelen, AL, White, M, van Sluijs, EMF, and Goodyer, IM. Diet Quality and Depressive Symptoms in Adolescence: No Cross-Sectional or Prospective Associations Following Adjustment for Covariates. Public Health Nutr (2018) 21(13):2376–84. doi:10.1017/S1368980018001179
33. Greenland, S. Quality Scores Are Useless and Potentially Misleading: Reply to “Re: A Critical Look at Some Popular Analytic Methods”. Am J Epidemiol (1994) 140(3):300–1. doi:10.1093/oxfordjournals.aje.a117250
34. McMartin, SE, Kuhle, S, Colman, I, Kirk, SF, and Veugelers, PJ. Diet Quality and Mental Health in Subsequent Years Among Canadian Youth. Public Health Nutr (2012) 15(12):2253–8. doi:10.1017/S1368980012000535
35. Kim, TH, Choiyoung, J, Lee, HH, and Park, Y. Associations between Dietary Pattern and Depression in Korean Adolescent Girls. J Pediatr Adolesc Gynecol (2015) 28(6):533–7. doi:10.1016/j.jpag.2015.04.005
36. Dreher, ML. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients (2018) 10(12):E1833. doi:10.3390/nu10121833
37. Liu, X, Yan, Y, Li, F, and Zhang, D. Fruit and Vegetable Consumption and the Risk of Depression: A Meta-Analysis. Nutrition (2016) 32(3):296–302. doi:10.1016/j.nut.2015.09.009
38. Molteni, R, Barnard, RJ, Ying, Z, Roberts, CK, and Gómez-Pinilla, F. A High-Fat, Refined Sugar Diet Reduces Hippocampal Brain-Derived Neurotrophic Factor, Neuronal Plasticity, and Learning. Neuroscience (2002) 112(4):803–14. doi:10.1016/s0306-4522(02)00123-9
39. Kim, YK, and Shin, C. The Microbiota-Gut-Brain axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr Neuropharmacol (2018) 16(5):559–73. doi:10.2174/1570159X15666170915141036
40. Taylor, AM, and Holscher, HD. A Review of Dietary and Microbial Connections to Depression, Anxiety, and Stress. Nutr Neurosci (2020) 23(3):237–50. doi:10.1080/1028415X.2018.1493808
41. Nguyen, B, Ding, D, and Mihrshahi, S. Fruit and Vegetable Consumption and Psychological Distress: Cross-Sectional and Longitudinal Analyses Based on a Large Australian Sample. BMJ Open (2017) 7(3):e014201. doi:10.1136/bmjopen-2016-014201
42. Best, O, and Ban, S. Adolescence: Physical Changes and Neurological Development. Br J Nurs (2021) 30(5):272–5. doi:10.12968/bjon.2021.30.5.272
43. Evans, GW, Li, D, and Whipple, SS. Cumulative Risk and Child Development. Psychol Bull (2013) 139(6):1342–96. doi:10.1037/a0031808
44. Molendijk, M, Molero, P, Ortuno Sanchez-Pedreno, F, Van der Does, W, and Martinez-Gonzalez, MA. Diet Quality and Depression Risk: a Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. J Affect Disord (2018) 226:346–54. doi:10.1016/j.jad.2017.09.022
45. Rothman, KJ, Greenland, S, and Lash, TL. Modern Epidemiology. Philadelphia, Pennsylvania, US: Wolters Kluwer Health/Lippincott Williams & Wilkins Philadelphia (2008).
46. Tennant, PW, Murray, EJ, Arnold, KF, Berrie, L, Fox, MP, Gadd, SC, et al. Use of Directed Acyclic Graphs (DAGs) to Identify Confounders in Applied Health Research: Review and Recommendations. Int J Epidemiol (2021) 50(2):620–32. doi:10.1093/ije/dyaa213
47. Reedy, J, Subar, AF, George, SM, and Krebs-Smith, SM. Extending Methods in Dietary Patterns Research. Nutrients (2018) 10(5):E571. doi:10.3390/nu10050571
48.Health Canada. Section 2: Foods and Beverages that Undermine Healthy Eating. Canada Food Guide (2022). Available from: https://food-guide.canada.ca/en/guidelines/section-2-foods-and-beverages-undermine-healthy-eating/ (Accessed July 6, 2022).
49. Georgiades, K, Duncan, L, Wang, L, Comeau, J, and Boyle, MH. Six-month Prevalence of Mental Disorders and Service Contacts Among Children and Youth in Ontario: Evidence from the 2014 Ontario Child Health Study. Can J Psychiatry (2019) 64(4):246–55. doi:10.1177/0706743719830024
50. Caine-Bish, NL, and Scheule, B. Gender Differences in Food Preferences of School-Aged Children and Adolescents. J Sch Health (2009) 79(11):532–40. doi:10.1111/j.1746-1561.2009.00445.x
Keywords: adolescents, youth, mental health and wellbeing, common mental disorders, healthy diet, vegetables and fruit, depression, anxiety
Citation: Dabravolskaj J, Marozoff S, Maximova K, Campbell S and Veugelers PJ (2022) Relationship Between Fruit and Vegetables Intake and Common Mental Disorders in Youth: A Systematic Review. Public Health Rev 43:1604686. doi: 10.3389/phrs.2022.1604686
Received: 12 December 2021; Accepted: 31 August 2022;
Published: 20 September 2022.
Edited by:
Diogo Costa, Bielefeld University, GermanyReviewed by:
Laura Orlando, University of Toronto, CanadaJune Kloubec, Bastyr University, United States
Copyright © 2022 Dabravolskaj, Marozoff, Maximova, Campbell and Veugelers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
PHR is edited by the Swiss School of Public Health (SSPH+) in a partnership with the Association of Schools of Public Health of the European Region (ASPHER)+
*Correspondence: Julia Dabravolskaj, dabravol@ualberta.ca
 Julia Dabravolskaj
Julia Dabravolskaj Shelby Marozoff1
Shelby Marozoff1 Katerina Maximova
Katerina Maximova