Abstract
Objective:
Amaranth, a nutritious iron source, is known for treating anemia in young children and lactating mothers, but its effectiveness in reducing hemoglobin concentration needs further investigation. Therefore, this study aimed to summarize the effectiveness of amaranth-based food interventions in improving hemoglobin concentration.
Method:
A randomized controlled trial and quasi-experimental study conducted since 2000 were searched in databases like PubMed, Scopus, Embase, Cochrane, AJOL, and Web of Science using prespecified keywords. Excel and Stata 17 were used for data extraction and analysis. Methodological quality was assessed using the JBI systematic review critical appraisal tool. Meta-analysis was done to estimate the overall intervention effect.
Result:
Ten studies were included from 1,032 articles (n = 1,225). The standardized mean hemoglobin concentration difference between groups was positive, with an overall effect of 0.08 (95%CI: −0.11, 0.26; p = 0.433), where I2 is 57.1%.
Conclusion:
The studies’ interventions showed positive effects on hemoglobin concentration, but their effectiveness was not statistically significant. This suggests the need for research on the impact of different cooking methods on iron bioavailability, phytic iron ratio, and intervention effects across different populations.
Systematic Review Registration:
Identifier PROSPERO CRD42023476402.
Introduction
Globally, the prevalence of anemia has been decreasing; however, it remains a public health concern, particularly among young children 40% (36%–44%) and pregnant women 36% (34%–39%) [1]. Anemia can result in various adverse outcomes, including adverse perinatal and birth outcomes, such as increased risk of postpartum hemorrhage, sepsis, growth and developmental issues in children, and even death in severe cases [2–4]. To reduce the burden of nutritional deficiency anemia, which is regarded as one of the top causes of anemia, various interventions have been implemented, such as iron supplementation, fortification and dietary interventions [5]. Among these dietary interventions, amaranth is recognized for its therapeutic effect on the treatment of anemia in young children and lactating mothers [6].
Amaranth, also known as Pseudocereal, which belongs to the genus Amaranthus, is an ancient crop with a rich history and cultivation in various parts of the world, and it is believed to have originated in South America [7]. There are various types of amaranth species, like Amaranthus caudatus L., A. cruentus L., and A. hypochondriacus Leucocarpus. These species were cultivated in different parts of America, including Mexico, Guatemala, and the Andrea region, as well as Asia and Africa [7–9]. Despite being discouraged for its use in ancient religious ceremonies, amaranth holds special religious significance in some regions of countries like India and Pakistan [10]. A few decades ago, its cultivation and utilization increased following the emergence of evidence regarding its nutritional value [11].
The production of grain amaranth, with a tiny seed less than 1 mm in diameter, outperforms corn in terms of yield per unit of land [7]. Its ability to thrive with efficient water use and adaptability makes it a crucial crop in the global food system by addressing the challenges of climate change and population growth. Besides the grains, amaranth’s leaves are edible and have spinach-like tastes [10, 12], though their chemical compositions differ from spinach. On average, amaranth comprises 61.3–76.5 g of crude carbohydrate, 13.1–21.5 crude protein (rich with lysine), 5.6–10.9 g crude fat and 2.7–5 g fiber per 100 g of amaranth. It also offers a balanced amino acid composition [12] and provides 7.61 mg of iron per 100 g of amaranth [13]. Moreover, amaranth leaves also provide 27.3 mg/100 g iron [14], which equals seven times more iron content than lettuce [15].
With its adaptability and nutritional composition, amaranth is a promising crop for combating malnutrition and food insecurity across the globe [11]. Amaranth can be combined with other cereals to produce different food products [10, 12], and its higher oil content makes it an alternative seed for different industries like oil production, pharmaceutics and cosmetics [16, 17]. The oxidative stability of the amaranth oil is even higher than that of commonly used sunflower oil. The oil content of raw A. caudatus L. and A. cruentus L. were 7.1% and 8.5%, with a high triacylglycerol (80.3%–82.3%) content [17]. Amaranth oil has been evidenced to have beneficial effects for patients with coronary heart disease and hypertension through reduction of total cholesterol, low-density lipoprotein and very low-density lipoprotein [18]. Such positive effects of this nutritious grain were also evidenced in its significant nutritional value [19–24].
Despite the abundance of literature suggesting the nutritional and therapeutic benefits of amaranth, its practical utilization for alleviating nutritional anemia demands further attention. This is highlighted by Anemia remaining a significant public health concern in key producers of amaranth, such as China, Kenya and several sub-Saharan African countries, where anemia ranges from 11% to 60% [8, 25–27]. The prevalence of nutritional anemia, even in amaranth-producing countries, raises questions about consumption patterns and the effectiveness of amaranth in reducing anemia. This underscores the limitation of evidence on the effectiveness of amaranth-containing foods on hemoglobin concentration. Additionally, while there are no previously published systematic reviews and meta-analysis to our knowledge on this specific topic, pre-identified empirical primary studies have shown opposing conclusions on the phenomenon [28, 29]. Therefore, this systematic review and meta-analysis aims to summarize the effectiveness of amaranth-based food products in improving hemoglobin concentration.
Methods
Study Desing
A systematic review and meta-analysis of primary studies was conducted in accordance with the Preferred Reporting Items for Systematic and Meta-Analysis-2020 (PRISMA-2020) guideline [30], as shown in Supplementary Material S1. The study also followed a pre-designed systematic review protocol that was registered in the International Prospective Register of Systematic Reviews (PROSPERO)—registration number CRD42023476402. Following the preliminary search, the scope of the review became narrowed to determining the effects of amaranth-containing food products on hemoglobin concentration.
Eligibility Criteria
As we aimed to assess the best available evidence related to the effectiveness of amaranth, interventional studies were targeted to be included in the review. Accordingly, a Randomized Controlled Trial (RCT), Quasi-Experimental Study (QE) and pre-post observational studies aimed to assess the effectiveness of amaranth-based food intervention on individuals aged 6 months or older regardless of their health condition were included in this review. To minimize bias in selecting studies [31], both published and unpublished studies available online from January 2000 to April 2024 were targeted in a comprehensive database search.
Additional eligibility criteria included population, intervention types, comparator and outcome. Amaranth-containing food can be consumed by different age groups, including young children and adults [22, 32]. Children older than 6 months should start complementary food [33]. Therefore, the population in this study consists of individuals over 6 months of age, regardless of their health status or any demographic characteristics. In terms of intervention type, studies involving food interventions that included amaranth as a key ingredient, regardless of specific food type, were targeted. The intervention could have been implemented in different settings like households, schools, and communities. Comparisons were made either against other functional foods or in the absence of any interventions for the control group.
The main outcome of this study was to determine the effect of amaranth on anemia, which is assessed by hemoglobin levels. Anemia’s diagnosis and severity are determined by the cut-off value of hemoglobin concentration, which is influenced by factors like age, sex, health status, and altitude [34]. Hence, studies that assessed the levels of hemoglobin in both the intervention and control groups were sought by measuring the mean and standard deviation of hemoglobin before and after the intervention. Then mean difference was generated to see the effect of the intervention on hemoglobin concentration, as recommended in the Cochrane Handbook [31].
In converse, studies assessing amaranth’s nutritive value, qualitative investigation, book chapters, compressive reviews, systematic reviews, studies that missed reporting hemoglobin before and after the intervention in both the intervention and control groups and studies published in another language other than English were excluded from the review.
Databases, Search Strategies and Study Selection
Online databases like PubMed, Scopus, Cochrane, Embase, Google Scholar, AJOL, and Web of Science were searched for the records. Furthermore, ProQuest’s dissertation and gray literature from search engines like Google were used to search unpublished studies. The initial search was done compressively using the following keywords: “effectiveness,” “efficacy,” “amaranth,” “amaranthus,” “amaranthus cruentus OR amaranthus caudatus OR amaranthus hypochondriacus,” “food OR snack,” and “intervention.” Then the search was updated by adding keywords like hemoglobin OR Anemia OR nutrition and by indexing dated between 1 January 2000 and 13 April 2024. The reference lists of eligible studies were also evaluated for potential articles based on selection criteria. The database search strategies used are provided as a supplementary document (Supplementary Material S2).
The bibliographies of articles identified from all databases were recorded and exported to EndNote [35] and stored in a respective database labelled group. MY and AE used the Covidence Systematic Review online software1 to independently review and screen articles by title, abstract and full text based on eligibility criteria. Any discrepancy was resolved by the third reviewer, AZ, and through discussion.
Methodological Quality
Methodological quality was assessed using the JBI systematic review critical appraisal tool for systematic review of effectiveness, which has a separate format to examine the quality of RCT and quasi-experimental studies [36]. To ensure the consistency of the data, the assessment was done independently by MY and AE using a table. In a situation where the design of the study was not clear, we decided on the protocol of the study, particularly based on randomization and the presence of a control group. Any discrepancy was resolved by discussing and reviewing the article together.
Data Collection, Analysis and Synthesis
Data were extracted from eligible studies in Excel using a pre-structure sheet by two independent reviewers (MY and AE) focusing on study design, year of publication, type of study, sample size, type of food intervention (drink, meal, snack, juice or else), part of amaranth used (leaves or grain), amaranth species, population, comparator food, follow up time, frequency of meal, statistical analysis, findings and conclusion. Any discrepancy was resolved by discussing and extracting the data together.
The characteristics of included studies, like the type of intervention, target population, the health status of the study participants, species, used amaranth part (leaves or grain), frequency of consumption and follow-up period, were synthesized and described in the table. Unreported standard deviation was obtained using the square root of n times the width of the confidence interval divided by 3.92 [29].
Meta-analysis was conducted using Stata version 17 to generate the pooled effect of amaranth-containing food on hemoglobin concentration. The standardized mean difference (Hedges’ g) was used to estimate the overall effect size considering variation in the population, intervention, and measurement of outcome. The pooled estimate was summarized and presented in a forest plot. A random effect model was used considering the characteristics of studies and observed heterogeneity. Publication bias was assessed using a funnel plot and Egger’s test. In response to a significant small study effect, trim-and-fill estimation was done and described using a trim-and-fill funnel plot [37]. A subgroup analysis was done to examine the source of heterogeneity.
Certainty of Evidence
Certainty of evidence and summary of findings on the effectiveness of amaranth-containing food on hemoglobin concentration was assessed using a Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach and the summary of findings table is presented as a supplementary document (Supplementary Material S3).
Result
Search Results and Study Selection
A systematic search of studies was conducted to access published and unpublished studies from databases like Embase, Scopus, PubMed, Cochrane and AJOL. Furthermore, Google Scholar and ProQuest were used for articles and dissertations. A total of 1,032 articles were found from database and citation search, and only 10 articles were found to fulfill the inclusion criteria from 70 overall records screened in full text, as depicted in Figure 1.
FIGURE 1
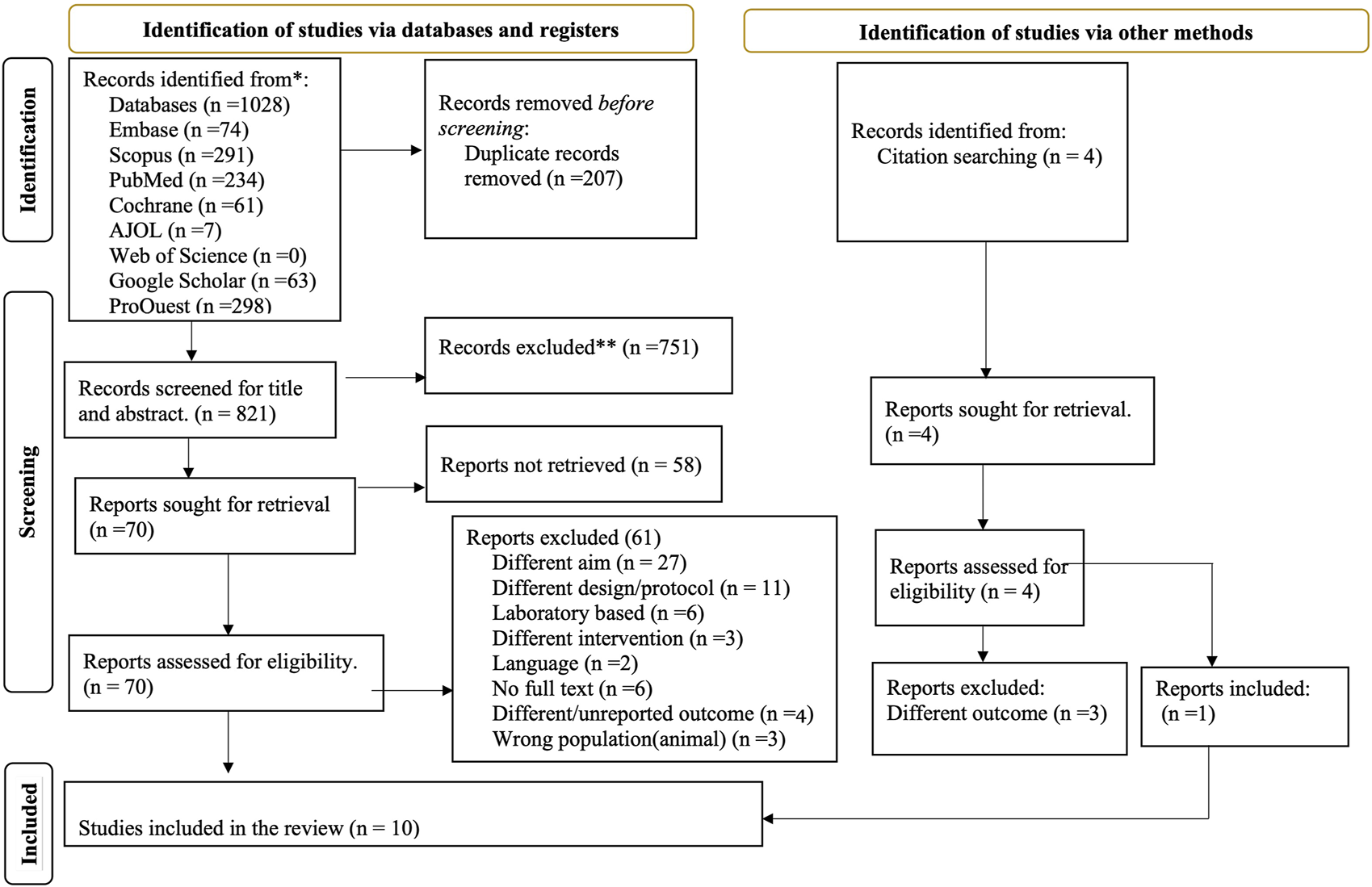
Preferred reporting items for systematic review and meta-analysis: the PRISMA flow diagram of screened, evaluated and included amaranth containing dietary intervention studies (Global systematic review, 2000–2024).
Methodological Quality of Included Studies
Methodological quality was assessed using the JBI systematic review critical appraisal tool for a systematic review of effectiveness review of RCT and QE studies [36]. The baseline characteristics of comparison groups in some of the QE studies were different in some of the included quasi-experiment study designs [38, 39]. The study design was not clearly specified as RCT or quasi-experimental [28, 39]. As a result, we considered the study design to be quasi-experimental design based on the study protocol. One QE study did not discuss the treatment other than the intervention given for the comparison group. Furthermore, the statistical analysis of the included studies was not appropriate to compare differences between groups (Table 1) [28].
TABLE 1
| Studies | Critical appraisal questions | Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | ||
| Ginting et al. [38] | Y | UC | Y | Y | Y | Y | Y | UC | Y | 7 |
| Nawiri et al. [39] | Y | UC | Y | Y | Y | UC | Y | Y | Y | 7 |
| Singh et al. [28] | Y | Y | UC | Y | Y | Y | Y | N | N | 6 |
| Muliani et al. [40] | Y | Y | Y | Y | Y | UC | Y | UC | Y | 7 |
| Total % of positive scores | 100 | 50 | 75 | 100 | 100 | 50 | 100 | 25 | 75 | |
Critical appraisal results for the included amaranth containing dietary intervention quasi-experimental studies (Global systematic review, 2000–2024).
JBI critical appraisal checklist for quasi-experimental studies (Y, yes; N, No; UC, Unclear; N/A, Not Applicable).
Q1, Is it clear in the study what is the “cause” and what is the “effect” (i.e., there is no confusion about which variable comes first)?
Q2, Were the participants included in any comparisons similar?
Q3, Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest?
Q4, Was there a control group?
Q5, Were there multiple measurements of the outcome both pre and post the intervention/exposure?
Q6, Was follow up complete and if not, were differences between groups in terms of their follow up adequately described and analyzed?
Q7, Were the outcomes of participants included in any comparisons measured in the same way?
Q8, Were outcomes measured in a reliable way?
Q9, Was appropriate statistical analysis used?
The assessment of the methodological quality of RCT showed that allocation concealment was only described by half of the included studies. Most studies did not report information regarding the blinding of the study of participants, treatment providers and outcome assessors [41–43]. One study discussed that all measurements were blind. However, the authors did not describe blinding participants, those delivering treatment and outcome assessors [44]. The baseline characteristics of study participants were also different in some of the studies [32, 42]. Regarding the statistical analysis, some studies did not report the approach they followed as an intention-to-treat or per protocol (Table 2) [41, 43, 45, 46]
TABLE 2
| Studies | Appraisal questions | Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | ||
| Fitriani et al. [43] | Y | UC | Y | UC | UC | Y | Y | Y | UC | Y | Y | Y | N | 8 |
| Konyole et al. [29] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13 |
| Macharia et al. [42] | Y | UC | N | UC | UC | UC | Y | Y | Y | Y | Y | Y | Y | 8 |
| Orsango et al. [32] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 12 |
| Stiller et al. [41] | Y | UC | Y | UC | N | UC | UC | Y | UC | Y | Y | N | Y | 6 |
| Hoevenet al. [44] | Y | Y | N | UC | UC | UC | Y | Y | Y | Y | Y | Y | Y | 9 |
| Total % of positive scores | 100 | 50 | 50 | 33.3 | 33.3 | 50 | 83.3 | 100 | 66.7 | 100 | 100 | 83.3 | 83.3 | |
Critical appraisal results for included amaranth containing dietary intervention randomized controlled trial studies (Global systematic review, 2000–2024).
JBI critical appraisal checklist for randomised controlled trials (Y, yes; N, No; UC, Unclear; N/A, Not Applicable).
Q1, Was true randomization used for assignment of participants to treatment groups?
Q2, Was allocation to treatment groups concealed?
Q3, Were treatment groups similar at the baseline?
Q4, Were participants blind to treatment assignment?
Q5, Were those delivering treatment blind to treatment assignment?
Q6, Were outcomes assessors blind to treatment assignment?
Q7, Were treatments groups treated identically other than the intervention of interest?
Q8, Was follow up complete and if not, were differences between groups in terms of their follow up adequately described and analyzed?
Q9, Were participants analyzed in the groups to which they were randomized?
Q10, Were outcomes measured in the same way for treatment groups?
Q11, Were outcomes measured in a reliable way?
Q12, Was appropriate statistical analysis used?
Q13, Was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial.
Characteristics of Included Studies
A total of ten studies were included, of which 60% them were RCT and 40% were QE studies. Most studies were published between 2012 and 2019 from five countries which are Indonesia [3], Kenya [3], South Africa [1], Ethiopia [1] and India (one published and one unpublished). About 30% of included studies were community based and 20% were school based. Most of the studies (70%) included a younger population, and one study included post-partum women without age specification. The total sample size was 1,225 (614 in the intervention and 611 in the control group), ranging from 20 to 334 and follow-up period from 2 to 72 weeks. Furthermore, about 70% of included studies used leaves of amaranths to prepare the intervention like juice, herbal drinks, porridge and bread (Table 3).
TABLE 3
| Study | S year | Study type | P year | Country | Design | Tn | In | Cn | Age | Health status | Follow (week) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fitriani et al. [43] | 2018 | Published | 2020 | Indonesia | SRCT | 128 | 64 | 64 | 18–60 years | Anaemic | 4 |
| Ginting et al. [38] | 2019 | Published | 2021 | Indonesia | QE | 30 | 15 | 15 | 20–40 years | Healthy | 2 |
| Konyole et al. [29] | 2013 | Published | 2019 | Kenya | PRCT | 334 | 167 | 167 | 6 months | Healthy | 36 |
| Macharia-Mutie et al. [42] | 2011 | Published | 2012 | Kenya | PRCT | 186 | 93 | 93 | 1–5 years | Healthy | 16 |
| Nawiri et al. [39] | NR | Published | 2013 | Kenya | QE | 107 | 56 | 51 | 2.5–6 years | Healthy | 13 |
| Orsango et al. [32] | 2017 | Published | 2020 | Ethiopia | CRCT | 100 | 50 | 50 | 2–5 years | Anaemic | 24 |
| Singh et al. [28] | NR | Published | 2023 | India | QE | 20 | 10 | 10 | 5–11 years | Anaemic | 6 |
| Stiller et al. [41] | 2016 | Unpublished | 2020 | India | CRCT | 123 | 58 | 65 | 6–39 months | Anaemic | 72 |
| Faber et al. [44] | 2012 | Published | 2015 | South Africa | PRCT | 167 | 86 | 81 | 6–12 years | Healthy | 12 |
| Muliani et al. [40] | UC | Published | 2017 | Indonesia | QE | 30 | 15 | 15 | .NR | Post-partum women | 2 |
| Study | Species | Used | Prepared food | Comparator | Consumption/week | Measurement | Conclusion |
|---|---|---|---|---|---|---|---|
| Fitriani et al. [43] | A. tricolor | leaves | Herbal drink | Iron supplement | 10 | NR | No significant difference |
| Ginting et al. [38] | A.tricolor | leaves | Juice (amaranth alone) | Beta Vulgaris juice | 5 | NR | Increase |
| Konyole et al. [29] | A.cruentus | grain | Complementary | CSB+ | NR | NR | Decrease |
| Macharia-Mutie et al., 2012 | not specified | grain | Porridge | Plain maize porridge | .NR | Sysmex hematological analyzer (KX-21, Sysmex) | No significant difference |
| Nawiri et al. [39] | not specified | leaves | Food | White cabbage | 5 | hemocue analyzer (Model B-Hemoglobin, Angelholm, Sweden) | Increase |
| Orsango et al. [32] | not specified | grain | Bread | Maize bread | 7 | HemoCue analyser 301 (Angelholm, Sweden) | Increase |
| Singh et al. [28] | not specified | leaves | Balls | Nothing | 14 | Haemoglobinometer (Fully Cell-Counter Automatic) | Increase |
| Stiller et al. [41] | A. tricolor | leaves | Cooked food | Nothing | 2 | HemoCue201+ | No significant difference |
| Faber et al. [44] | A.cruentus | leaves | Cooked food | School meal | 5 | Coulter® Ac·T™ 5diff CP | No significant difference |
| Muliani et al. [40] | A. tricolor | leaves | Red spinach extract tablet (amaranth alone) | Iron capsule | 21 | NR | Increase |
Characteristics of included amaranth containing dietary intervention studies (Global systematic review, 2000–2024).
S Year: NR is for study year not reported, UC: unclear; Design: SRCT: Standard RCT, QE: quasi experimental, PRCT: Parallel RCT, and CRCT: Cluster RCT; Tn: Total sample size; In: sample size for intervention group; Cn: Sample size for control group: CSB+: fortified corn–soy blend plus.
Effectiveness of Amaranth-Containing Food on Improving Hemoglobin Level
The minimum standardized mean difference for the effect of amaranth-containing food on hemoglobin was zero (95%CI: −0.29, 0.29) [42] and the largest effect size was 1.46 (0.51, 2.41) [28]. The forest plot indicates the presence of heterogeneity where I2 is 57.1%. To investigate the possible reason behind observed variation, subgroup analysis was conducted, and it showed that there is a higher heterogeneity among studies that recruited anemic subjects (86.02%) than healthy subjects (no heterogeneity). Similarly, heterogeneity was higher among quasi-experimental studies (75.3%) than RCT (36.3%). Studies that used amaranth leaves showed a higher heterogeneity (71.7%) than those that used amaranth grain (67.3%).
The standardize mean hemoglobin concentration difference between the intervention and control groups found to be positive, with the overall pooled effect of amaranth-containing food on hemoglobin concentration being 0.08 (95% CI: −0.11, 0.26). The statistical test of the overall effect size is not different from zero (p-value 0.4330). The studies by Singh et al. [28] and Muliani et al. [40] may affected the findings with a low sample size and large effect size (Figure 2). To identify the most influential studies, a leave-one-out graph was constructed, and it was found that omitting Muliani et al. [40] and Singh et al. [28] decreased the overall effect size by 0.07.
FIGURE 2
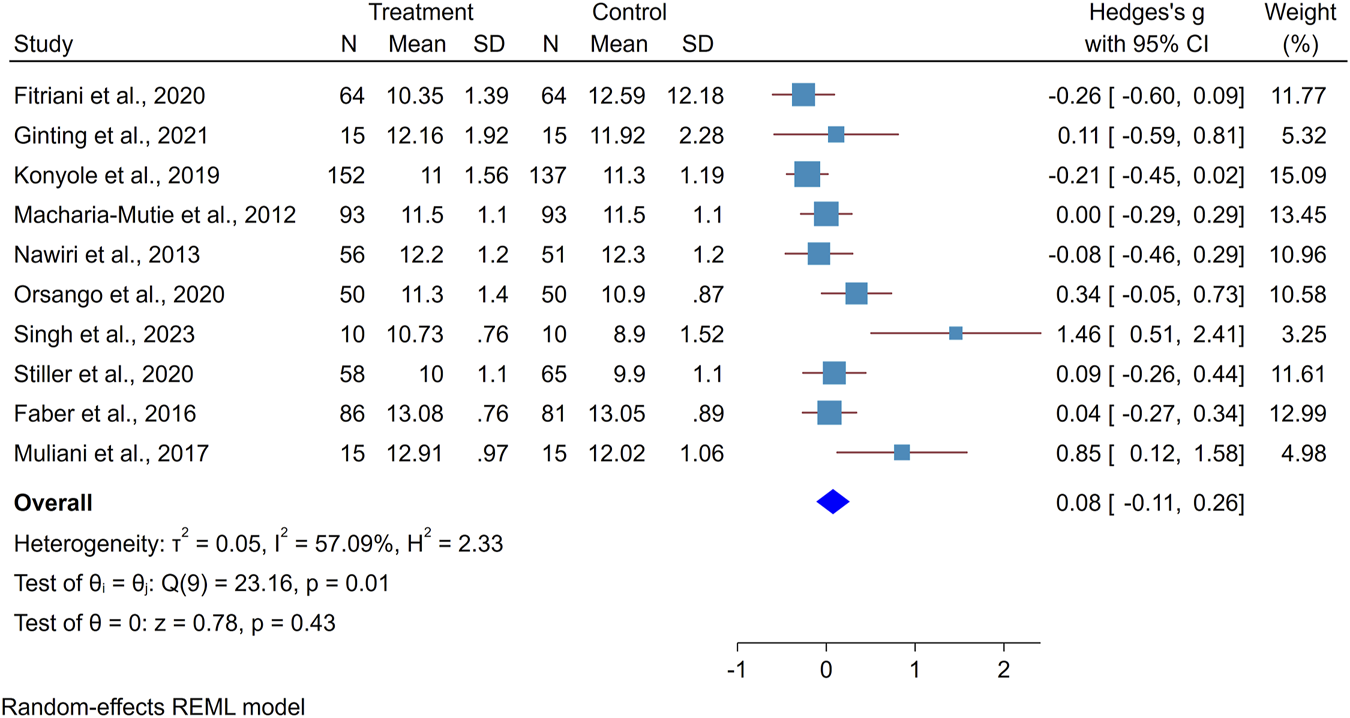
Forest plot showing the effect of amaranth containing dietary intervention on hemoglobin level (Global systematic review and metanalysis, 2000–2024).
Assessment of Bias
The asymmetric distribution of the study in the funnel plot indicated the presence of publication bias (Supplementary Material S4). Furthermore, egger’s test of small study effect (p-value < 0.001) suggests the presence of a small study effect. The trim-and-fill method which used to correct publication bias by imputing two studies where only one falls within alpha < 0.05. The trim-and-fill estimator changes the direction and magnitude of the overall effect size −0.01 (95%CI: −0.32, 0.3) (Supplementary Material S4).
Subgroup Analysis
The effect of the intervention was compared across different groups such as study design, population and amaranth part used. Accordingly, the result showed a significant positive effect of the intervention for postpartum women, 0.85 (95%CI; 0.12, 1.58) (Figure 3).
FIGURE 3
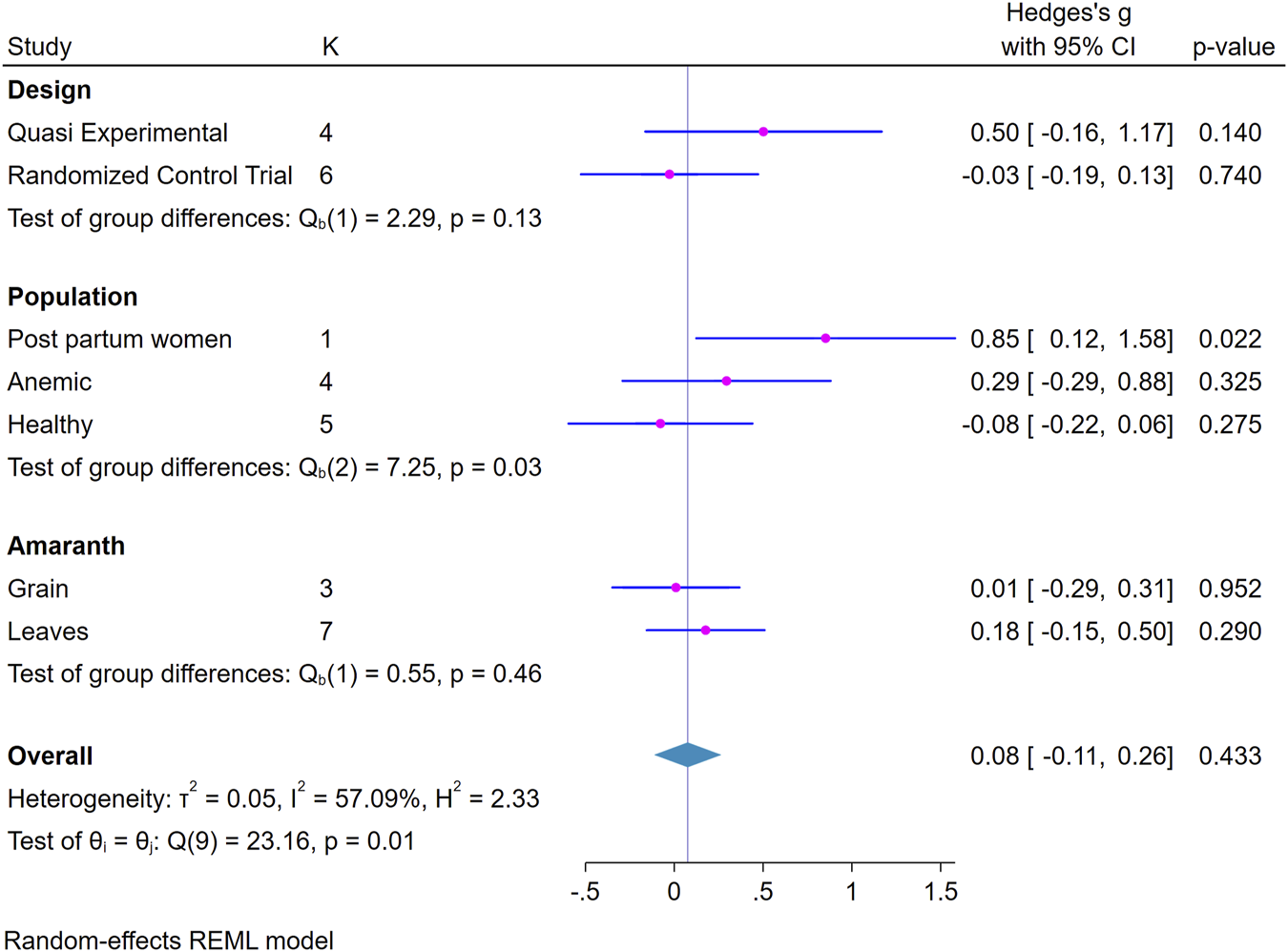
Forest plot showing subgroup analysis of the effect of amaranth containing dietary intervention on hemoglobin level (Global systematic review and metanalysis, 2000–2024).
Discussion
In this systematic review and meta-analysis, we summarized the effectiveness of amaranth-containing food interventions on the concentration of hemoglobin as reported in primary studies. The pooled standardized mean difference showed that the interventions were not found to have a significant effect on hemoglobin concentration. However, it was found to have a significant effect on postpartum women. This might be due to post-partum women having a 20%–37% risk of iron deficiency anemia during pregnancy could start to return back to the pre-pregnancy state parallel to the physiological changes during postpartum [47]. On the other hand, although factors like failure to exclusive breastfeeding and multiparity were known to be associated with the occurrence of anemia [48], which could be partly mediated by the preexisting physiologic anemia during pregnancy and childbirth due to enhanced demands and blood loss [49]; the provision of amaranth-containing foods could serve as an important source of nutritional intervention to alleviate nutritional anemia for postpartum women. The effectiveness of such interventions could be influenced by factors such as the target population, the type of food prepared, the method of preparation, the frequency of consumption, and the inclusion of deworming.
To ultimately utilize the high content of iron in amaranth, avoidance of the factors that inhibit iron absorption, like deworming, and reducing intake of caffeine-containing drinks like coffee and tea are indispensable [50]. However, only three included studies [32, 38, 39] reported considering these factors during intervention provision. These studies found a significant positive effect of the intervention following providing anthelmintic medication to the participants prior to the intervention [32, 39] and provided clear instructions about food that should be avoided during the intervention period [38]. This underscores the importance of deworming for improved outcomes following nutritional intervention, as also suggested elsewhere [51]. Likewise, an amaranth-based nutritional intervention should prioritize managing the consumption of coffee and determine of phytic iron ratio. The failure to deworm and limit caffeine consumption in most of the reviewed studies [28, 40, 41, 43, 44, 52] could partly explain the lack of significance of amaranth-containing foods in enhancing hemoglobin concentration.
In addition to limiting previous consumption habits like restricting caffeine intake and deworming against intestinal parasites, which reduce iron absorption, the recipe preparation methods are also essential. Though amaranth is the best source of iron [13] and its inclusion in the diet improves the nutrient content of the food [53], the preparation method could affect the concentration of phytate and bioavailability of iron [54]. Different studies have reported methods of preparation of food that reduce the phytate content of amaranth, like soaking, germinating and fermentation [32, 55]. It is evidenced that boiling [54], popping and toasting [56] decrease iron, whereas soaking, germination [57], and fermentation [56] increase iron contents of amaranth. Moreover, germination decreases the concentration of phytic acid from amaranth [58]. On the contrary to the high content of iron, the presence of antinutrients like phytate could be the reason for the insignificant effect of amaranth-based food, as only two studies took measures of reducing phytate and supporting the micronutrient bioavailability [29, 32]. This might be due to the absorption of iron affected by the concentration of phytate [59]. The observed non-significant intervention effect could be linked to these preparation methods issues, highlighting the importance of taking these measures before preparing amaranth-containing food to maximize iron availability and absorption. Furthermore, various amaranth-containing foods were provided in combination with other functional grains or leaves, which could influence the bioavailability and absorption as their contents’ negative effects were not controlled.
This systematic review is the first to summarize the effect of amaranth-containing dietary intervention on improving hemoglobin concentration. The comprehensive and updated searches that ensure the inclusion of all relevant studies are among the strengths of this review. The main limitation of this review is the heterogeneity of included studies in terms of factors like the health status of the population, age variation, type of intervention, and frequency of consumption. Such inconsistency and the observed publication bias may underestimate the overall effect of the intervention. Therefore, further studies on amaranth-based dietary intervention in different contexts and target populations should be conducted. Furthermore, there was no clear information across the study about the ratio of amaranth in the prepared intervention. Which also affects the findings and conclusion of the review. Bias related to allocation concealment and blinding could also affect the treatment effect in either way. Therefore, an experimental study with the application of all the standard procedures is needed to assess the effect of amaranth-based dietary intervention. Another potential issue could be the loss of studies that might have been included if we had incorporated other languages. However, we acknowledge this limitation, considering that most databases index abstracts of studies written in non-English languages. Therefore, we believe the number of studies lost due to this limitation is minimal.
Conclusion
Amaranth-containing foods could serve as a potential source of iron to help increase hemoglobin levels among postpartum women. However, for other groups, we didn’t find strong evidence supporting a significant increase in hemoglobin concentration following dietary intervention with amaranth. Despite its high iron content, amaranth-containing food interventions appeared to have little or no effect on improving hemoglobin concentrations. Factors such as the phytic-to-iron ratio in the food and preparation method may have affected the bioavailability of iron in primary studies. Therefore, future research should investigate the impact of different cooking methods on iron bioavailability and the phytic-to-iron ratio to standardize preparation methods and enhance the effectiveness of amaranth-containing food in raising hemoglobin concentrations. Recommendations for improving the effectiveness of amaranth-food consumption should include deworming, avoiding caffeine intake, and reducing phytate content through soaking, germination, and fermentation. Meanwhile, boiling, popping, and toasting should be avoided, as they reduce the iron content of amaranth-containing foods. This study also underscores the need for further investigation of the intervention effect in different populations.
Statements
Author contributions
MY was involved in the study’s conception, while all authors contributed to protocol development, screening, review, and data extraction. MY conducted the formal analysis, interpretation, and manuscript drafting, while all authors participated in data curation, validation, review and approval of the final draft. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/phrs.2024.1607597/full#supplementary-material
Abbreviations
RCT, Randomized Controlled Trial; QE, Quasi Experimental Study; PICOS, Population, Intervention, Comparison, Outcome and Study design.
Footnotes
References
1.
StevensGAPaciorekCJFlores-UrrutiaMCBorghiENamasteSWirthJPet alNational, Regional, and Global Estimates of Anaemia by Severity in Women and Children for 2000-19: A Pooled Analysis of Population-Representative Data. Lancet Glob Health (2022) 10(5):e627–e639. 10.1016/S2214-109X(22)00084-5
2.
The WOMAN-2 trial collaboratorsWOMAN-2 trial collaborators. Maternal Anaemia and the Risk of Postpartum Haemorrhage: A Cohort Analysis of Data From the WOMAN-2 Trial. Lancet Glob Health (2023) 11(8):e1249–e1259. 10.1016/S2214-109X(23)00245-0
3.
World Health Organization. Nutritional Anaemias: Tools for Effective Prevention and Control (2017).
4.
MurrayCJLAravkinAYZhengPAbbafatiCAbbasKMAbbasi-KangevariMet alGlobal Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. The lancet (2020) 396(10258):1223–49. 10.1016/s0140-6736(20)30752-2
5.
MithraPKhatibMNSinhaAPKumarNHollaRUnnikrishnanBet alInterventions for Addressing Anemia Among Children and Adolescents: An Overview of Systematic Reviews. Front Pediatr (2020) 8:549549. 10.3389/fped.2020.549549
6.
JoshiNVermaKC. A Review on Nutrition Value of Amaranth (Amaranthus Caudatus L.): The Crop of Future. J Pharmacognosy Phytochemistry (2020) 9(4):1111–3.
7.
JonesVH. The Grain Amaranths: A Survey of Their History and Classification. Jonathan Deininger Sauer. Ann Mo Bot Garden (1950) 37. pp. 561-632, plates 10-14. American Antiquity. 2017;19(1):90-2. 10.2307/2394403
8.
GrazianoSAgrimontiCMarmiroliNGullìM. Utilisation and Limitations of Pseudocereals (Quinoa, Amaranth, and Buckwheat) in Food Production: A Review. Trends Food Sci and Technology (2022) 125:154–65. 10.1016/j.tifs.2022.04.007
9.
AderibigbeOREzekielOOOwoladeSOKoreseJKSturmBHenselO. Exploring the Potentials of Underutilized Grain Amaranth (Amaranthus spp.) Along the Value Chain for Food and Nutrition Security: A Review. Crit Rev Food Sci Nutr (2022) 62(3):656–69. 10.1080/10408398.2020.1825323
10.
AdhikaryDKhatri-ChhetriUSlaskiJ. Amaranth: An Ancient and High-Quality Wholesome Crop. In: VidurangaYW, editor. Nutritional Value of Amaranth. Rijeka: IntechOpen (2020). Ch. 4.
11.
Weerasekara ACWaisundara VY. Amaranth as a Pseudocereal in Modern Times: Nutrients, Taxonomy, Morphology and Cultivation. In: VidurangaYW, editor. Nutritional Value of Amaranth. Rijeka: IntechOpen (2020). Ch. 1.
12.
MlakarSGTurinekMJakopMBavecMBavecF. Nutrition Value and Use of Grain Amaranth: Potential Future Application in Bread Making. Agricultura (2009) 6(4):43–53.
13.
Soriano-GarcíaMSaraid Aguirre-DíazI. Nutritional Functional Value and Therapeutic Utilization of Amaranth. In: VidurangaYW, editor. Nutritional Value of Amaranth. Rijeka: IntechOpen (2020). Ch. 7.
14.
GuptaAVermaTVermaS. Prevention of Anaemia With Underutilized Green Leafy Vegetables (2024).
15.
SrivastavaR. Nutritional Quality of Some Cultivated and Wild Species of Amaranthus L. Int J Pharm Sci Res (2011) 2(12):3152.
16.
Nasirpour-TabriziPAzadmard-DamirchiSHesariJPiravi-VanakZ. Amaranth Seed Oil Composition. In: VidurangaYW, editor. Nutritional Value of Amaranth. Rijeka: IntechOpen (2020). Ch. 9.
17.
GamelTHMesallamASDamirAAShekibLALinssenJP. Characterization of Amaranth Seed Oils. J Food Lipids (2007) 14(3):323–34. 10.1111/j.1745-4522.2007.00089.x
18.
MartirosyanDMMiroshnichenkoLAKulakovaSNPogojevaAVZoloedovVI. Amaranth Oil Application for Coronary Heart Disease and Hypertension. Lipids Health Dis (2007) 6:1–511X. (Electronic)):1. 10.1186/1476-511X-6-1
19.
Avila-NavaAAlarcon-TelesforoSLTalamantes-GomezJMCoronaLGutierrez-SolisALLugoRet alDevelopment of a Functional Cookie Formulated with Chaya (Cnidoscolus Aconitifolius (Mill.) I.M. Johnst) and Amaranth (Amaranthus Cruentus). Molecules (2022) 27(21):7397. 10.3390/molecules27217397
20.
CotovanuIStroeSGUrsachiFMironeasaS. Addition of Amaranth Flour of Different Particle Sizes at Established Doses in Wheat Flour to Achieve a Nutritional Improved Wheat Bread. Foods (2022) 12(1):133. 10.3390/foods12010133
21.
JanNHussainSZNaseerBBhatTA. Amaranth and Quinoa as Potential Nutraceuticals: A Review of Anti-nutritional Factors, Health Benefits and Their Applications in Food, Medicinal and Cosmetic Sectors. Food Chem X (2023) 18:100687. 10.1016/j.fochx.2023.100687
22.
OkothJKOcholaSAGikonyoNKMakokhaA. Development of a Nutrient-Dense Complementary Food Using Amaranth-Sorghum Grains. Food Sci Nutr (2017) 5(1):86–93. 10.1002/fsn3.367
23.
Perez-RamirezIFSotelo-GonzalezAMLopez-EchevarriaGMartinez-MaldonadoMA. Amaranth Seeds and Sprouts as Functional Ingredients for the Development of Dietary Fiber, Betalains, and Polyphenol-Enriched Minced Tilapia Meat Gels. Molecules (2022) 28(1):117. 10.3390/molecules28010117
24.
Skwaryło-BednarzBStępniakPMJamiołkowskaAKopackiMKrzepiłkoAKlikockaH. The Amaranth Seeds as a Source of Nutrients and Bioactive Substances in Human Diet. Acta Scientiarum Polonorum-hortorum Cultus (2020) 19:153–64. 10.24326/asphc.2020.6.13
25.
OkiroEAJosephNKGitongaCWSnowRW. Anaemia Among Kenyan Children: A Call for Improved Monitoring and Intervention in School-Aged Children. Trans R Soc Trop Med Hyg (2020) 114(8):627–31. 10.1093/trstmh/traa032
26.
LemoineATounianP. Childhood Anemia and Iron Deficiency in Sub-saharan Africa - Risk Factors and Prevention: A Review. Arch Pediatr (2020) 27(8):490–6. 10.1016/j.arcped.2020.08.004
27.
ZhuZSudfeldCRChengYQiQLiSElhoumedMet alAnemia and Associated Factors Among Adolescent Girls and Boys at 10–14 Years in Rural Western China. BMC Public Health (2021) 21(1):218. 10.1186/s12889-021-10268-z
28.
SinghRChandnaKJainNSinghR. A Study on the Development and Effectiveness of Iron-Rich Balls From Underutilized Leaf Source for Anaemia Management in Children (2023).
29.
KonyoleSOOmolloSAKinyuruJNSkauJKHOwuorBOEstambaleBBet alEffect of Locally Produced Complementary Foods on Fat-free Mass, Linear Growth, and Iron Status Among Kenyan Infants: A Randomized Controlled Trial. Matern Child Nutr (2019) 15(4):e12836. 10.1111/mcn.12836
30.
PageMMcKenzieJEBossuytPMBoutronIHoffmannTCMulrowCDet alThe PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Bmj (2021) 372(71):790–9. 10.1016/j.rec.2021.07.010
31.
HigginsJPThomasJChandlerJCumpstonMLiTPageMJet alCochrane Handbook for Systematic Reviews of Interventions. John Wiley and Sons (2019).
32.
OrsangoAZLohaELindtjornBEngebretsenIMS. Efficacy of Processed Amaranth-Containing Bread Compared to Maize Bread on Hemoglobin, Anemia and Iron Deficiency Anemia Prevalence Among Two-To-Five Year-Old Anemic Children in Southern Ethiopia: A Cluster Randomized Controlled Trial. PLoS One (2020) 15(9):e0239192. 10.1371/journal.pone.0239192
33.
World Health Organization. Complementary Feeding 2023 (2024). Available from: https://www.who.int/health-topics/complementary-feeding#tab=tab_1 (Accessed: October 24, 2023).
34.
World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity Switzerland2011 (2024). Available from: https://iris.who.int/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf (Accessed: December 30, 2023).
35.
The EndNote Team. EndNote. EndNote. 20 ed. Philadelphia, PA: Clarivate (2013).
36.
TufanaruCMunnZAromatarisECampbellJHoppL. Chapter 3: Systematic Reviews of Effectiveness. In: AromatarisEMunnZ, editors. JBI Manual for Evidence Synthesis2020 (2024).
37.
ShiLLinL. The Trim-And-Fill Method for Publication Bias: Practical Guidelines and Recommendations Based on a Large Database of Meta-Analyses. Medicine (Baltimore) (2019) 98(23):e15987. 10.1097/MD.0000000000015987
38.
GintingRSamosirFJHartonoVTHOngkoDAnjaniDPurwaningrumDet alThe Intake of Beta Vulgaris and Amaranthus Tricolor L. Juice on Increasing the Hemoglobin Level. 2021.
39.
NawiriMPNyambakaHMurungiJI. Sun-dried Cowpeas and Amaranth Leaves Recipe Improves Beta-Carotene and Retinol Levels in Serum and Hemoglobin Concentration Among Preschool Children. Eur J Nutr (2013) 52(2):583–9. 10.1007/s00394-012-0360-2
40.
MulianiRHSoejoenoesASuherniTHadisaputroSMashoediID. Effect of Consuming Red Spinach (Amaranthus Tricolor L) Extract on Hemoglobin Level in Postpartum Mothers. Belitung Nurs J (2017) 3(4):432–7. 10.33546/bnj.156
41.
StillerCKGolembiewskiSKEStuetzWGolembiewskiMRinconDGMondalSet alEffect of Diversified Meals Only, or Either Enriched With Amaranthus Tricolor and Moringa Oleifera Leaf Powder or Commercial Micronutrient Powder on Prevention/treatment of Anemia in Adivasi Children. West Bengal, India (2020).
42.
Macharia-MutieCWMorettiDVan den BrielNOmusundiAMMwangiAMKokFJet alMaize Porridge Enriched With a Micronutrient Powder Containing Low-Dose Iron as NaFeEDTA But Not Amaranth Grain Flour Reduces Anemia and Iron Deficiency in Kenyan Preschool Children. J Nutr (2012) 142(9):1756–63. 10.3945/jn.112.157578
43.
FitrianiUNoviantoFWijayantiETriyonoA. Effectiveness of Herbs Containing Curcuma Xanthorrhiza, Elephantopus Scaber L and Amaranthus Tricolor L in Iron Deficiency Anemia Patients. Biodiversitas J Biol Divers (2020) 21(5). 10.13057/biodiv/d210560
44.
van der HoevenMFaberMOseiJKrugerASmutsCM. Effect of African Leafy Vegetables on the Micronutrient Status of Mildly Deficient Farm-School Children in South Africa: A Randomized Controlled Study. Public Health Nutr (2016) 19(5):935–45. 10.1017/S1368980015002037
45.
NambogweE. Effect of Amaranth Porridge Supplementation on the Nutritional and Health Status of Children 6-59 Months in Uganda. Makerere University (2022).
46.
EgbiGGlover-AmengorMTohouenouMMZotorF. Contribution of Amaranthus Cruentus and Solanum Macrocarpon Leaves Flour to Nutrient Intake and Effect on Nutritional Status of Rural School Children in Volta Region, Ghana. J Nutr Metab (2020) 2020. 10.1155/2020/1015280
47.
DavidsonEMScoullarMJLPeachEMorganCJMelepiaPOpiDHet alQuantifying Differences in Iron Deficiency-Attributable Anemia During Pregnancy and Postpartum. Cell Rep Med (2023) 4(7):101097. 10.1016/j.xcrm.2023.101097
48.
FatimaSSameenaMaharTMahjabeenSoomroSUN. Factors Associated With Postpartum Iron Deficiency Anemia. Pakistan J Med and Health Sci (2022) 16(10):348–50. 10.53350/pjmhs221610348
49.
HorowitzKMIngardiaCJBorgidaAF. Anemia in Pregnancy. Clin Lab Med (2013) 33(2):281–91. 10.1016/j.cll.2013.03.016
50.
ShubhamKAnukiruthikaTDuttaSKashyapAVMosesJAAnandharamakrishnanC. Iron Deficiency Anemia: A Comprehensive Review on Iron Absorption, Bioavailability and Emerging Food Fortification Approaches. Trends Food Sci and Technology (2020) 99:58–75. 10.1016/j.tifs.2020.02.021
51.
GirumTWasieA. The Effect of Deworming School Children on Anemia Prevalence: A Systematic Review and Meta-Analysis. The open Nurs J (2018) 12:155–61. 10.2174/1874434601812010155
52.
Macharia-MutieCWMorettiDVan den BrielNOmusundiAMMwangiAMKokFJet alMaize Porridge Enriched With a Micronutrient Powder Containing Low-Dose Iron as NaFeEDTA but Not Amaranth Grain Flour Reduces Anemia and Iron Deficiency in Kenyan Preschool Children. J Nutr (2012) 142(9):1756–63. 10.3945/jn.112.157578
53.
TibagonzekaJWJMuyindaAMNakimbugweDMuyongaJH. Acceptability and Nutritional Contribution of Grain Amaranth Recipes in Uganda. Ajfand (2014) 14(3):8979–97. 10.18697/ajfand.63.13015
54.
Marquez-MolinaOXochitl Lopez-MartinezL. Effect of Various Process Conditions on the Nutritional and Bioactive Compounds of Amaranth. In: VidurangaYW, editor. Nutritional Value of Amaranth. Rijeka: IntechOpen (2020). Ch. 8.
55.
KinyuruJNKonyoleSOOnyango-OmoloSAKenjiGMOnyangoCAOwinoVOet alNutrients, Functional Properties, Storage Stability and Costing of Complementary Foods Enriched With Either Termites and Fish or Commercial Micronutrients. J Insects as Food Feed (2015) 1(2):149–58. 10.3920/jiff2014.0011
56.
AmareEMouquet-RivierCRochetteIAdishAHakiGD. Effect of Popping and Fermentation on Proximate Composition, Minerals and Absorption Inhibitors, and Mineral Bioavailability of Amaranthus Caudatus Grain Cultivated in Ethiopia (2016). p. 0022–1155.
57.
ThakurPKumarKAhmedNChauhanDEainHRQUJanSet alEffect of Soaking and Germination Treatments on Nutritional, Anti-Nutritional, and Bioactive Properties of Amaranth (Amaranthus Hypochondriacus L.), Quinoa (Chenopodium Quinoa L.), and Buckwheat (Fagopyrum Esculentum L.). Curr Res Food Sci (2021) 4:917–25. 10.1016/j.crfs.2021.11.019
58.
De RuizACBressaniR. Effect of Germination on the Chemical Composition and Nutritive Value of Amaranth Grain. Cereal Chem (1990) 67(6):519–22.
59.
HurrellRFReddyMBJuilleratMACookJD. Degradation of Phytic Acid in Cereal Porridges Improves Iron Absorption by Human Subjects. Am J Clin Nutr (2003) 77(5):1213–9. 10.1093/ajcn/77.5.1213
Summary
Keywords
amaranthus, hemoglobin, undernutrition, intervention, systematic review
Citation
Yilma MT, Eifa A, Belayneh M and Orsango AZ (2025) Effect of Amaranth-Containing Dietary Intervention in Improving Hemoglobin Concentration: A Systematic Review and Meta-Analysis. Public Health Rev 45:1607597. doi: 10.3389/phrs.2024.1607597
Received
02 June 2024
Accepted
09 December 2024
Published
03 January 2025
Volume
45 - 2024
Edited by
Raquel Lucas, University Porto, Portugal
Reviewed by
Ratna C. Purwestri, Czech University of Life Sciences Prague, Czechia
Kritika Gupta, University of Mississippi, United States
Updates
Copyright
© 2025 Yilma, Eifa, Belayneh and Orsango.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. PHR is edited by the Swiss School of Public Health (SSPH+) in a partnership with the Association of Schools of Public Health of the European Region (ASPHER)+
*Correspondence: Mekdes Tigistu Yilma, mekdestg@outlook.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.