Identifying causes of death, let alone COVID-19-specific mortality, is a challenge in many low- and middle-income countries. Lack of testing and large numbers of community deaths without a physician to medically certify the cause of death, are barriers to knowing the full impact of the pandemic on mortality [1].
Verbal Autopsy (VA) is a technique for determining the most medically likely causes of death in the community, where no physician is available to complete a medical certificate of cause of death. Briefly, VA uses a structured questionnaire to elicit the signs and symptoms exhibited by the deceased in the period before death and that can reliably be understood by and reported on by family members and other lay caregivers [2]. The pattern of responses to the VA questionnaire is used by physicians or a computer algorithm to assign the most probable cause of death [3, 4].
As part of the development of the Rapid Mortality Surveillance Technical Package, the WHO VA Reference Group produced a short questionnaire and algorithm, the InterVA CRMS model [5], to distinguish deaths due to COVID-like illness (CLI) from deaths due to other natural and unnatural causes. The algorithm produces estimates of the probability of death being associated with CLI, based on the answers in the short questionnaire.
This study aims to evaluate the performance of the InterVA CRMS model against ultrasound guided-minimally invasive autopsy, which is the best available reference during the pandemic. To our knowledge, no study with this purpose has been carried out yet.
Setting
Sao Paulo is the largest city in Brazil, with more than 12 million inhabitants. It has been one of the epicenters of COVID-19, having reached in March 2021 more than 8,300 case notifications in a single day [6].
The Autopsy Service at the University of Sao Paulo (SVOC-USP) performs autopsies of natural deaths in the city of Sao Paulo for deaths without an established cause of death. On March 20th, 2020, the Governor of the State of Sao Paulo decreed emergency measures for the prevention of contagion by SARS-CoV-2, suspending conventional autopsies within the State. Since then, only a few ultrasound-guided minimally invasive autopsies (MIA-US) have been performed at the SVOC-USP.
Approach
We applied the short questionnaire and algorithm to all the 112 deaths occurring from March to December 2020 that underwent MIA-US at the SVOC-USP.
The MIA-US procedure has been described by Duarte-Neto et al. (2020) and Dolhnikoff al. (2020) [7, 8]. Briefly, internal organs were visualized using a portable ultrasound and tissue sampling was performed using Tru-Cut© semi-automatic 14G needles. Our protocol includes extensive sampling of lungs, heart, liver, kidneys, spleen, testis, skin, skeletal muscle, bone marrow, salivary glands, brain, and intestines. Reverse-transcription polymerase chain reaction (RT-PCR) was employed for molecular detection of SARS-CoV-2 in oropharyngeal swabs or pulmonary tissue as previously described [7, 9]. A team of health professionals, extensively trained in techniques appropriate to the grieving environment, asked the COVID-19 specific questions to families/caregivers after they signed the Consent Form.
Results
According to the MIA-US, 72 deaths had COVID-19 as the cause of death (positive group), and 40 individuals died from other causes (negative group). In the positive group, 39 (54.2%) were male (mean age 54.1 ± 19.1). In the negative group, 16 (40.0%) were male (mean age 61.2 ± 21.1).
Table 1 shows the frequencies and percentages of each sign and symptom included in the short questionnaire in the positive and negative groups, and the sensitivity and specificity of each sign/symptom.
TABLE 1
| Signal/Symptoma | Positive n (%) (N = 72) | Negative n (%) (N = 40) | Sensitivityb (95% Confidence interval) | Specificityb (95% Confidence interval) |
|---|---|---|---|---|
| Difficulty breathing | — | — | 91.7 (82.7–96.9) | 47.5 (31.5–63.9) |
| yes | 66 (91.7) | 20 (50) | — | — |
| no | 5 (6.9) | 19 (47.5) | — | — |
| don´t know | 1 (1.4) | 1 (2.5) | — | — |
| Fatigue | — | — | 79.2 (68–87.8) | 42.5 (27–59) |
| yes | 57 (79.2) | 23 (57.5) | — | — |
| no | 8 (11.1) | 17 (42.5) | — | — |
| don´t know | 7 (9.7) | 0 | — | — |
| Fever | — | — | 75 (63.4–84.6) | 82.5 (67.2–92.7) |
| yes | 54 (75) | 7 (17.5) | — | — |
| no | 16 (22.2) | 33 (82.5) | — | — |
| don´t know | 2 (2.8) | 0 | — | — |
| Positive test | — | — | 72.2 (60.4–95.1) | 80 (64.4–90.9) |
| yes | 52 (72.2) | 0 | — | — |
| no | 7 (9.7) | 32 (80) | — | — |
| don´t know | 13 (18.1) | 8 (20) | — | — |
| Cough | — | — | 66.7 (54.6–77.3) | 67.5 (50.9–81.4) |
| yes | 48 (66.7) | 13 (32.5) | — | — |
| no | 23 (31.9) | 27 (67.5) | — | — |
| don´t know | 1 (1.4) | 0 | — | — |
| Contact COVID-19 | — | — | 34.7 (23.9–46.8) | 80 (64.4–90.9) |
| yes | 25 (34.7) | 2 (5) | — | — |
| no | 32 (44.4) | 32 (80) | — | — |
| Don’t know | 15 (20.8) | 6 (15) | — | — |
| Loss smell/taste | — | — | 18.1 (10–28.9) | 80 (64.4–90.9) |
| yes | 13 (18.1) | 6 (15) | — | — |
| no | 47 (65.3) | 32 (80) | — | — |
| Don’t know | 12 (16.7) | 2 (5) | — | — |
Frequency and percentage of each sign and symptom in the groups classified as COVID-19 positive or negative by the Ultrasound-guided Minimally Invasive Autopsy - COVID-19 Case-Control study in Brazil project, Sao Paulo, Brazil, March - December 2020.
All the answers about living in an area with social distancing/stay-at-home measures were “Yes”, those about injuries were “No”, and those about traveling to a region where COVID-19 was present were “Don't know”.
Sensitivity was calculated here as the proportion of presence of a signal/symptom (answer “Yes”) in the group classified as positive by Ultrasound-guided Minimally Invasive Autopsy. Specificity was calculated as the proportion absence of signal/symptom (answer “No”) in the group classified as negative by Ultrasound-guided Minimally Invasive Autopsy.
The probabilities of death by COVID-19 predicted by the InterVA CRMS algorithm (Figure 1) have high variability in the negative group while those in the positive group tend to concentrate on higher values. The cutoff value for the probability of death obtained from a ROC curve was 0.89. A sensitivity of 0.83 and a specificity of 0.88 are associated with this cutoff. The area under the ROC curve is 0.90.
FIGURE 1
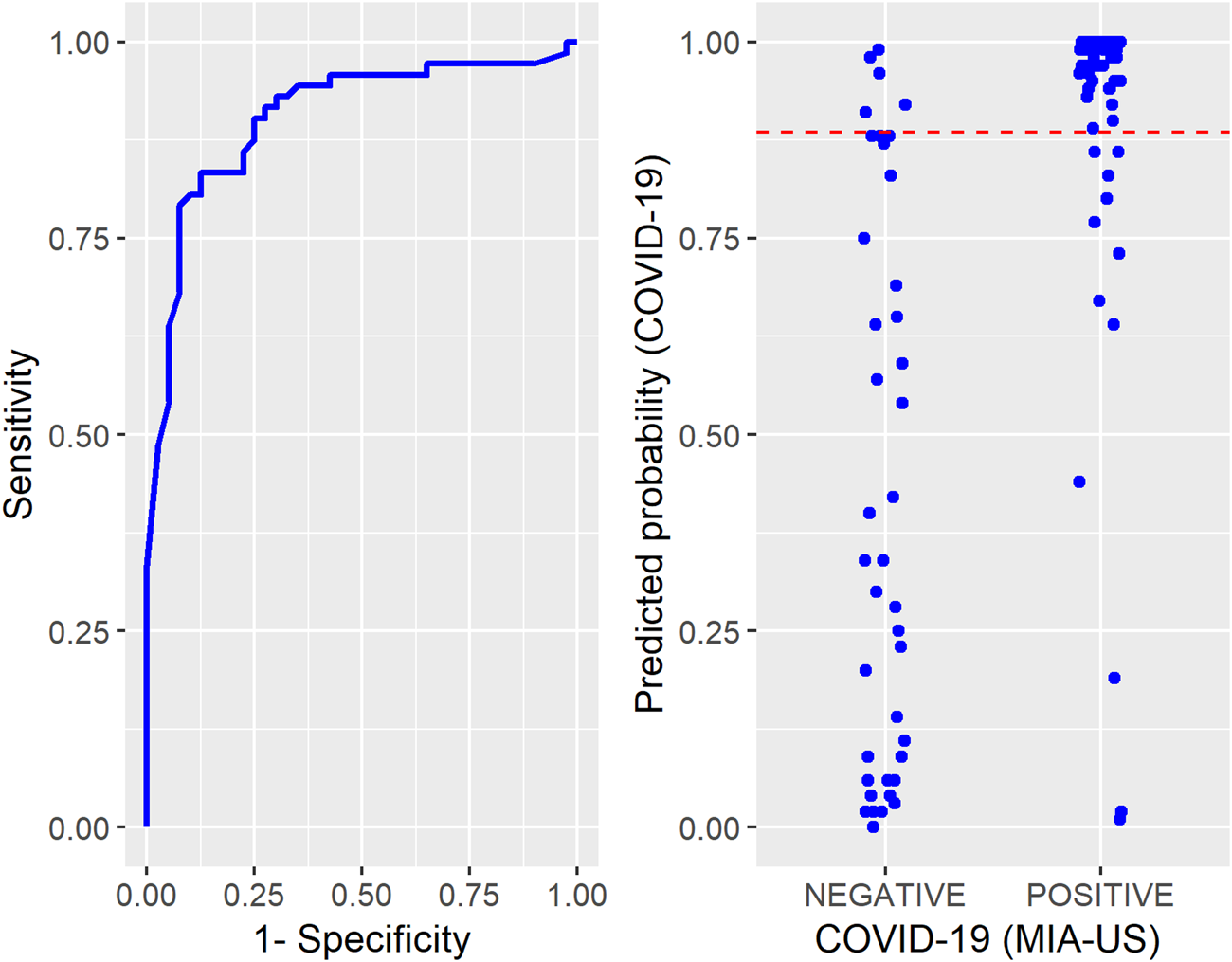
Left: Receiver Operating Characteristic Curve constructed to determine the cutoff for the probabilities of COVID-19 predicted by the InterVA CRMS algorithm. Right: Individual values of the predicted probabilities of COVID-19 according to the Ultrasound-guided Minimally Invasive Autopsy classification (positive or negative). The dashed line in red represents the cutoff - COVID-19 Case-Control study in Brazil project, Sao Paulo, Brazil, March - December 2020.
MIA-US attributed COVID-19 as the cause of death to four pediatric cases, but all of them had probabilities of death due to COVID-19 smaller than the cutoff 0.89 and were classified as negative for COVID-19 by the InterVA CRMS algorithm according to the procedures described above. Excluding all cases below 20 years-old from the sample (4 pediatric cases just mentioned and one classified as negative by MIA-US), the same cutoff value was obtained from the ROC curve (area under curve = 0.93) and this was associated with a sensitivity of 0.88 and a specificity of 0.87.
Discussion
Our results show that InterVA CRMS is a reliable way to rapidly track mortality due to CLI in adults with high sensitivity and specificity. The application of a questionnaire like this, quick and easy to understand, followed by the use of an algorithm for reading the results, which requires minimum computer capacity, can help to count cases of death by CLI in communities without extensive testing and medical certification of cause of death. In addition, initiatives such as this have their role even in large urban centers with well-established autopsy services, since most of them have had their activities disrupted during the pandemic due to the risk of contagion of staff members.
Adjustments to the questionnaire and algorithm are likely to be needed, not only to better discriminate the disease among children, but also as the epidemic progresses and as more knowledge is accrued. For example, the inclusion of a question of whether the decedent had been vaccinated for COVID-19 would be helpful for the cause of death assignment. Once large and representative samples of InterVA CRMS data with vaccination status are made available, they could be used to assess fatal vaccine failure.
Conclusion
Although more validation studies are needed, our findings indicate that COVID-19 deaths can be correctly assigned in adults using a simple set of questions about their signs and symptoms, helping in directing control measures during the course of the pandemic.
Statements
Ethics statement
This study has been approved by the School of Medicine, University of São Paulo, Review Board for Human Studies. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
PS and FMS: study design -CS, LB, AB: statistical analysis -PA: control of the data aquisition -PS, AD-N, LD, MD, JTF, RAAM: MIA-US -CSM, TL, JTF, RAAM: Interviews All authors wrote and read the manuscript.
Funding
This work was funded by Bill and Melinda Gates Foundation (INV-002396), Conselho Nacional de Desenvolvimento Científico e Tecnológico (401825/2020-5) and is, in part, and output of the Bloomberg Philanthropies Data for Health Initiative (https://www.bloomberg.org/public-health/strengthening-health-data/). The views expressed are not necessarily those of the Philanthropies.
Acknowledgments
This paper was written in honor of Peter Byass, who developed the CRMS algorithm and died on Aug 16, 2020. We thank Cassia Arruda, Kely Cristina Soares Bispo, Lisie Tocci Justo e Reginaldo Silva do Nascimento, for technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Vital Strategies, World Health Organization. Revealing the Toll of COVID-19: A Technical Package for Rapid Mortality Surveillance and Epidemic Response. New York: Vital Strategies (2021). Available at: https://www.who.int/publications/i/item/revealing-the-toll-of-covid-19(Accessed February 15, 2021).
2.
WHO. Verbal Autopsy Standards: Ascertaining and Attributing Causes of Death (2016). Available at: https://www.who.int/healthinfo/statistics/verbalautopsystandards/en/(Accessed February 15, 2021).
3.
SerinaPRileyIStewartAJamesSLFlaxmanADLozanoRet alImproving Performance of the Tariff Method for Assigning Causes of Death to Verbal Autopsies. BMC Med (2015) 13:291. 10.1186/s12916-015-0527-9
4.
McCormickTHLiZRCalvertCCrampinACKahnKClarkSJProbabilistic Cause-Of-Death Assignment Using Verbal Autopsies. J Am Stat Assoc (2016) 111:1036–49. 10.1080/01621459.2016.1152191
5.
Byass. InterVA–software for Verbal Autopsy (2021). Available at: http://www.byass.uk/interva/crms 2020(Accessed February 15, 2021).
6.
SEADE Foundation. SP Contra O Novo Coronavirus – Boletim Completo (2021). Available at: https://www.seade.gov.br/coronavirus/ (Accessed April 10, 2021).
7.
Duarte-NetoANMonteiroRAAda SilvaLFFMalheirosDMACde OliveiraEPTheodoro-FilhoJet alPulmonary and Systemic Involvement in COVID-19 Patients Assessed with Ultrasound-Guided Minimally Invasive Autopsy. Histopathology (2020) 77:186–97. 10.1111/his.14160
8.
DolhnikoffMFerreira FerrantiJde Almeida MonteiroRADuarte-NetoANSoares Gomes-GouvêaMViu DegaspareNet alSARS-CoV-2 in Cardiac Tissue of a Child with COVID-19-Related Multisystem Inflammatory Syndrome. Lancet Child Adolesc Health (2020) 4:790–4. 10.1016/s2352-4642(20)30257-1
9.
CormanVMLandtOKaiserMMolenkampRMeijerAChuDKet alDetection of 2019 Novel Coronavirus (2019-nCoV) by Real-Time RT-PCR. Euro Surveill (2020) 25(3):1. 10.2807/1560-7917.ES.2020.25.3.2000045
Summary
Keywords
mortality, surveillance, COVID-like illness, minimally invasive autopsy, InterVA CRMS
Citation
Duarte-Neto AN, Marinho MdF, Barroso LP, Saldiva de André CD, da Silva LFF, Dolhnikoff M, Afonso de André P, Minto CM, de Moura CS, Leite TF, Filho JT, Monteiro RAdA, Setel P, Bratschi MW, Mswia R, Saldiva PHN and Bierrenbach AL (2021) Rapid Mortality Surveillance of COVID-19 Using Verbal Autopsy. Int J Public Health 66:1604249. doi: 10.3389/ijph.2021.1604249
Received
16 May 2021
Accepted
15 September 2021
Published
05 October 2021
Volume
66 - 2021
Edited by
Nino Kuenzli, Swiss Tropical and Public Health Institute (Swiss TPH), Switzerland
Reviewed by
Pedro Souza, Death Verification Service Dr. Rocha Furtado, Brazil
Updates
Copyright
© 2021 Duarte-Neto, Marinho, Barroso, Saldiva de André, da Silva, Dolhnikoff, Afonso de André, Minto, de Moura, Leite, Filho, Monteiro, Setel, Bratschi, Mswia, Saldiva and Bierrenbach.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carmen D. Saldiva de André, carmensaldiva@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.