Abstract
Objectives: We tended to explore the association of indoor air pollution (IAP) and non-neoplastic digestive system diseases (NNDSD) among the Chinese middle-aged and older population.
Methods: From 2011 to 2018, we included 7884 NNDSD-free adults from the China Health and Retirement Longitudinal Study (CHARLS). Physician-diagnosed NNDSD was obtained by self-reported information at baseline and updated across follow-up surveys. We investigated the associations between baseline exposure of solid fuel use for cooking and/or heating and NNDSD diagnosed during follow-up through Cox proportional hazard models. Furthermore, we examined the relationship between cooking fuel switching and NNDSD diagnosed during follow-up.
Results: Solid fuel use for cooking and/or heating was positively associated with NNDSD after adjusting for potential confounders. The risk of NNDSD among subjects who always use solid fuel for cooking (adjusted hazard ratio [aHR]: 1.42; 95% confidence interval [CI]: 1.09, 1.84) was higher than those with always clean fuels. Moreover, we found a lower NNDSD risk among participants who switched from solid to clean cooking fuel (aHR: 0.65; 95% CI: 0.49, 0.87) than those with always solid fuels.
Conclusion: Our present study shows that indoor solid fuel use is a dependent risk factor for NNDSD. Moreover, switching to clean fuel may contribute to the prevention of digestive system illnesses.
Introduction
Exposure to indoor air pollution (IAP) ranks the top ten leading risks for diseases worldwide [1, 2]. Approximately 2.5 billion people worldwide are exposed to IAP from cooking with solid fuels, and the number increases when including those heating and lighting with solid fuels [3]. Incomplete combustion of solid fuels in developing countries becomes a major source of exposure to IAP (4). Solid fuel-related pollutants mainly include polycyclic aromatic hydrocarbons, particulate matter, nitrous oxide, carbon monoxide, and sulfur dioxide, which are two to three-fold higher in indoor environments than outdoors. The use of solid fuels is a particularly pressing issue in China [4]. Although the proportion of people exposed to indoor air pollution from solid fuels declines, 32% of the Chinese population still use solid fuels for cooking or heating [4]. It is estimated that 271,089 (209,882 to 346,561) deaths in China were attributable to solid fuel use in 2017(3).
Digestive tract disorders have become global diseases with accelerating increased incidence in countries whose societies have become westernized, like China [5, 6]. Moreover, evidence has shown that some non-neoplastic digestive system diseases (NNDSD), such as helicobacter pylori infection and chronic gastritis, are precursors to the development of digestive cancers [7–9]. Identifying potential risk factors for NNDSD may help prevent the development of NNDSD and gastrointestinal tumors. Previous epidemiological investigations have reported that exposure to ambient pollutants, such as ozone, contributes to a higher risk of digestive diseases [10–14]. However, the effect of IAP caused by solid fuel on NNDSD is incompletely understood with an epidemiological gap. Moreover, past studies on the relationship between air pollution and digestive diseases mainly focused on short-term exposure to specific pollutants [11, 13].
Hence, to partly provide epidemiological evidence for this topic, we assessed whether long-term chronic exposure to cooking and heating solid fuel use separately or simultaneously is associated with non-neoplastic digestive system diseases. We also explored whether the risk of NNDSD in participants who have switched from solid to clean fuel was lower compared to always using solid fuel.
Methods
Study Population
The current study was based on the China Health and Retirement Longitudinal Study (CHARLS), aiming to collect high-quality data representative of middle-aged and elderly people in China [15]. All participants underwent an interview to collect data on sociodemographic characteristics, health status, and medical history. The CHARLS baseline survey was conducted in 2011, with regular follow-up visits every 2–3 years in 2013, 2015, and 2018, respectively. All participants provided written informed consent during the investigation. A detailed description is available on its website (http://charls.pku.edu.cn/en).
17,708 adults were recruited in 2011. We excluded participants who had been diagnosed of non-neoplastic digestive system diseases (NNDSD) in 2011 and before (n = 4717), participants with missing data on indoor fuel source (n = 1540), diagnosing NNDSD (n = 2789) and demographic characteristics (n = 778); then 7,884 participants were left for the baseline analysis of the use of cooking and heating fuel types and their combined effects on NNDSD. For the follow-up analysis, we additionally excluded 4922 participants with missing data on cooking fuel type, resulting in 2962 individuals for analysis (Figure 1). Since data on heating fuel in 2015 were not available, we analyzed the effect of switching fuel types on the risk of NNDSD only regarding cooking fuel data.
FIGURE 1
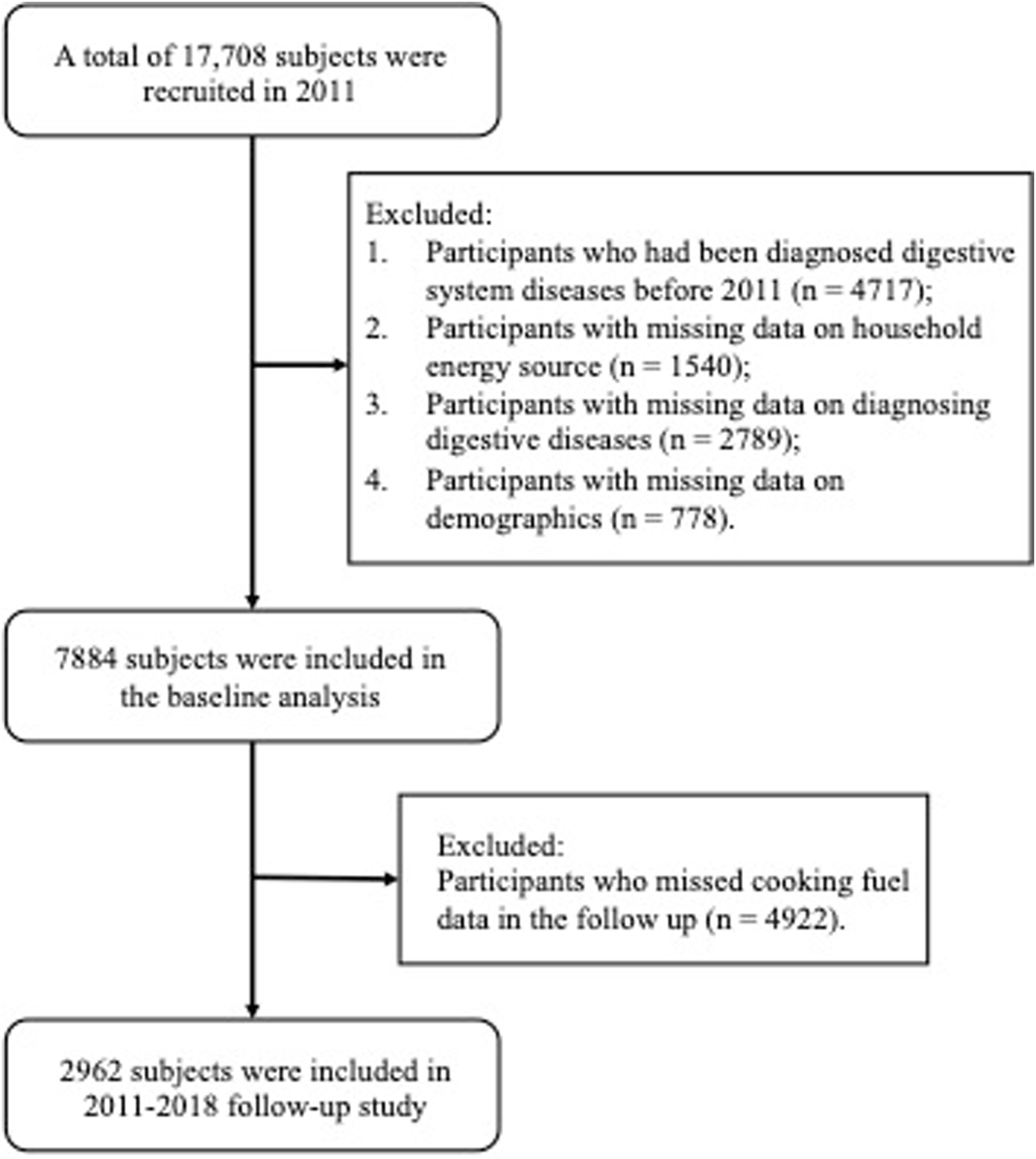
Flowchart of the selection process of population (China Health and Retirement Longitudinal Study, China, 2011, 2013, 2015, 2018).
Outcome of Interest
Physician-diagnosed NNDSD after 2011 was defined as the outcome of interest. Physician-diagnosed NNDSD was obtained from a self-reported questionnaire across follow-up surveys (“Have you been diagnosed with stomach or other non-neoplastic digestive system diseases by a doctor?”). If the subject’s answer to this question was “Yes”, he or she was defined as suffering from NNDSD. The survival time was defined as the time from the baseline date to the dates of the NNDSD diagnosis, loss to follow-up, or the end of follow-up (2018), whichever occurred first.
Indoor Energy Source
We dichotomized cooking fuels and heating fuels [clean fuels (natural gas, marsh gas, liquefied petroleum gas, solar, electric, or concentration heating) vs. solid fuels (coal, crop residues, wood-burning, or others)]. Notably, heating fuel information use was not available in 2015–2016. Therefore, we evaluated the effect of fuel type conversion on NNDSD only with the cooking fuel data in the follow-up analysis.
We divided the population into four groups in the follow-up analysis based on the type of fuel used at baseline and updated across follow-up visits: always solid fuel, always clean fuel, solid-to-clean fuel, and clean-to-solid fuel. The NNDSD emerged after fuel type conversion. For example, a subject who was diagnosed with NNDSD firstly in 2013, used solid energy in 2011 and clean energy in 2013, would be classified into the solid-to-clean fuel group; a subject who was a clean fuel user in 2011 while solid fuel user in 2015, diagnosed with NNDSD firstly in 2015, would be divided into clean-to-solid fuel group. Other groups were defined similarly.
Covariates
Covariates adjusted in our analyses included sex, age (middle-aged adults, 45–65 years; old adults, >65 years), educational level (illiteracy or informal education; elementary school or above), marital status (married, unmarried), smoking status (smokers, non-smokers), residence region (city or town; village), self-reported economic level (relatively poor or poor, average, relatively high or high), hypertension comorbidity (yes, no), diabetes (yes, no), dyslipidemia (yes, no), cardiovascular disease (yes, no), stroke (yes, no), liver diseases (yes, no), lung diseases (yes, no), cancer or malignant tumor (yes, no), and kidney diseases (yes, no).
The educational level was obtained by question, “what’s the highest level of education you have attained now?” Then, the answers were classified into two groups (illiteracy or informal education, elementary school or above). Marital status was divided into married and unmarried. Non-smokers were defined as those who had never smoked, and smokers were defined as those who had smoked or were currently smoking. Systolic blood pressure (SBP) or diastolic blood pressure (DBP) over 140/90 mmHg was defined as hypertension. Diabetes was defined as [1]: had been diagnosed as diabetes by a clinical doctor [2], fasting blood glucose (FBG) >200 mg/dl (11.1 mmol/L). HDL-C, LDL-C, glucose, and triglyceride were obtained from blood examination. Cardiovascular disease, stroke, liver diseases, lung diseases, cancer or malignant tumors, and kidney diseases were defined as previously diagnosed with this disease by a clinical doctor. Cardiovascular diseases included heart attack, coronary heart disease, angina, congestive heart failure, and other problems.
Statistical Analyses
Descriptive statistical analysis was used to compare population characteristics according to the indoor fuel types at baseline and fuel type change patterns during follow-up. The categorical variables were presented as frequency (proportions).
We performed Cox proportional hazards regression models to calculate adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) for the association between indoor solid fuel and NNDSD. We evaluated the proportional hazards assumption using the accumulated martingale residuals and found no obvious violation of the proportional hazards assumption [16]. In the baseline analysis, we estimate the relationship between cooking or heating fuel use, separately or simultaneously, and the risk of NNDSD among 7,884 participants. In the follow-up analysis, we evaluated the association between cooking fuel type conversion and the risk of NNDSD among 2962 participants. We conducted stratified analyses by baseline characteristics including age, sex, hypertension, and smoking status with the fully adjusted model.
Three models were fitted: Model 1 was a crude model; Model 2 adjusted for age, sex, and educational level; Model 3 was a fully adjusted model with additional adjustment for marital status, smoking status, residence region, self-reported economic level, hypertension comorbidity, cardiovascular disease, stroke, liver diseases, lung diseases, cancer or malignant tumor and kidney diseases.
We performed sensitivity analyses based on our primary Cox models. Firstly, we analyzed the association of household fuels conversion and NNDSD among non-smokers. Secondly, we performed stratified analyses by baseline characteristics to assess whether they could be effect modifiers of the studied association, including age, gender, smoking, and hypertension. Thirdly, we also performed sub-analyses with additional adjustment for population weighting concentrations of PM2.5 () and geographical weighting concentrations of PM2.5. Fourthly, we examined the associations between household fuel exposure at baseline and NNDSD stratified by comorbidities using Cox models.
All analyses were performed by SAS version 9.4 (SAS Institute, Cary, NC, United States), and R 4.1.1. A two-sided test p < 0.05 was considered statistically significant.
Results
Basic Characteristics of the Study Population
The baseline characteristics of the participants in the baseline and follow-up analyses are shown according to the indoor fuel patterns (Table 1). We included 7,884 and 2962 enrolled participants in the baseline and follow-up analyses. Solid fuel was used for cooking by 3,709 (47.04%) participants and for heating by 3,732 (47.34%) participants. Solid cooking and heating fuel users were more inclined to be older, have families in poor financial conditions, be more likely to be smokers, and be illiterate or informally educated. 803 (27.11%) participants always used solid fuel during the follow-up period, 1,352 (45.6%) always used clean fuel, and 176 (5.94%) used fuel types that have switched from clean to solid fuel. Compared with participants who always used solid fuel, subjects who always used clean fuel and changed from solid to clean fuel tended to be females, aged from 45 to 65 years old, unmarried, and have a relatively high education level and high economic level at baseline.
TABLE 1
| Characteristics | Household fuel use at baseline | Cooking fuel changes during follow up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cooking | Heating | Always clean | Solid to clean | Clean to solid | Always solid | p | |||||
| Clean | Solid | p | Clean | Solid | p | ||||||
| N | 4175(53.0) | 3709(47.0) | 4152(52.7) | 3732(47.3) | 1,352(45.6) | 631(21.3) | 176(5.9) | 803(27.1) | |||
| Sex | 0.982 | 0.982 | 0.194 | ||||||||
| Male | 2034(48.7) | 1808(48.8) | 2024(48.8) | 1818(48.7) | 634(46.9) | 279(44.22) | 83(47.2) | 401(49.9) | |||
| Female | 2141(51.3) | 1901(51.2) | 2128(51.2) | 1914(51.3) | 718(53.1) | 352(55.8) | 93(52.8) | 402(50.1) | |||
| Age | <0.001 | <0.001 | <0.001 | ||||||||
| 45–65 | 3375(80.8) | 2716(73.2) | 3338(80.4) | 2753(73.8) | 992(73.4) | 410(65.0) | 122(69.3) | 537(66.9) | |||
| >65 | 800(19.2) | 993(26.8) | 814(19.6) | 979(26.2) | 360(26.6) | 221(35.0) | 54(30.7) | 266(33.1) | |||
| Region | <0.001 | <0.001 | <0.001 | ||||||||
| City/town | 3669(87.9) | 3662(98.7) | 3701(89.1) | 3630(97.3) | 1,182(87.4) | 615(97.5) | 170(96.6) | 796(99.1) | |||
| Village | 506(12.1) | 47(1.3) | 451(10.9) | 102(2.7) | 170(12.6) | 16(2.5) | 6(3.4) | 7(0.9) | |||
| Smoking | <0.001 | 0.061 | 0.010 | ||||||||
| Smokers | 1,544(37.0) | 1,522(41.0) | 1,574(37.9) | 1,492(40.0) | 538(39.8) | 276(43.7) | 65(36.9) | 372(46.3) | |||
| Non-smokers | 2631(63.0) | 2187(59.0) | 2578(62.1) | 2240(60.0) | 814(60.2) | 355(56.3) | 111(63.1) | 431(53.7) | |||
| Marital status | 0.535 | 0.014 | 0.399 | ||||||||
| Married | 3720(89.1) | 3321(89.5) | 3742(90.1) | 3299(88.4) | 1,027(76.0) | 481(76.2) | 140(79.6) | 632(78.7) | |||
| Unmarried | 455(10.9) | 388(10.5) | 410(9.9) | 433(11.6) | 325(24.0) | 150(23.8) | 36(20.4) | 171(21.3) | |||
| Economy standard | <0.001 | <0.001 | <0.001 | ||||||||
| Poor | 1,600(38.3) | 1751(47.2) | 1,627(39.2) | 1724(46.2) | 539(39.8) | 301(47.7) | 74(42.1) | 390(48.6) | |||
| Average | 2435(58.3) | 1874(50.5) | 2395(57.7) | 1914(51.3) | 752(55.7) | 309(50.0) | 99(56.2) | 399(46.7) | |||
| High | 140(3.4) | 84(2.3) | 130(3.1) | 94(2.5) | 61(4.5) | 21(3.3) | 3(1.7) | 14(1.7) | |||
| Education | <0.001 | <0.001 | <0.001 | ||||||||
| Illiteracy or informal education | 1,483(35.5) | 2052(55.3) | 1,533(36.9) | 2002(53.6) | 488(36.1) | 344(54.5) | 87(49.4) | 504(62.8) | |||
| Elementary school or above | 2692(64.5) | 1,657(44.6) | 2619(63.1) | 1730(46.4) | 864(63.9) | 287(45.5) | 89(50.6) | 299(37.2) | |||
| Hypertension | 0.060 | <0.001 | 0.312 | ||||||||
| Yes | 955(22.9) | 783(21.1) | 990(23.8) | 748(20.0) | 334(24.7) | 146(23.1) | 42(23.9) | 170(21.2) | |||
| No | 3220(77.1) | 2926(78.9) | 3162(76.2) | 2984(80.0) | 1,018(75.3) | 485(76.9) | 134(76.1) | 633(78.8) | |||
| Liver disease | 0.631 | 0.891 | 0.200 | ||||||||
| Yes | 119(2.9) | 99(2.7) | 116(2.8) | 102(2.7) | 42(3.1) | 21(3.3) | 1(0.6) | 21(2.6) | |||
| No | 4056(97.1) | 3610(97.3) | 4036(97.2) | 3630(97.3) | 1,310(96.9) | 610(96.7) | 175(99.4) | 782(97.4) | |||
| Lung disease | 0.001 | 0.019 | 0.415 | ||||||||
| Yes | 292(7.0) | 333(9.0) | 301(7.3) | 324(8.7) | 107(7.9) | 57(9.0) | 14(8.0) | 80(10.0) | |||
| No | 3883(93.0) | 3376(91.0) | 3851(92.7) | 3408(91.3) | 1,245(92.1) | 574(91.0) | 162(92.0) | 723(90.0) | |||
| Cancer or malignant tumor | 0.133 | 0.209 | 0.707 | ||||||||
| Yes | 40(1.0) | 24(0.7) | 39(0.9) | 25(0.7) | 10(0.7) | 4(0.6) | 2(1.1) | 4(0.5) | |||
| No | 4135(99.0) | 3685(99.3) | 4113(99.1) | 3707(99.3) | 1,342(99.3) | 627(99.4) | 174(98.9) | 799(99.5) | |||
| Heart disease | 0.684 | <0.001 | 0.273 | ||||||||
| Yes | 355(8.5) | 305(8.2) | 401(9.7) | 259(6.9) | 128(9.5) | 53(8.4) | 9(5.1) | 71(8.8) | |||
| No | 3820(91.5) | 3404(91.8) | 3751(90.3) | 3473(93.1) | 1,224(90.5) | 578(91.6) | 167(94.9) | 732(91.2) | |||
| Stroke | 0.867 | 0.276 | 0.027 | ||||||||
| Yes | 78(1.89) | 67(1.8) | 83(2.0) | 62(1.7) | 30(2.2) | 21(3.3) | 2(1.1) | 9(1.1) | |||
| No | 4097(98.1) | 3642(98.2) | 4069(98.0) | 3670(98.3) | 1,322(97.8) | 610(96.7) | 174(98.9) | 794(98.9) | |||
| Kidney disease | 0.156 | 0.066 | 0.408 | ||||||||
| Yes | 188(4.5) | 193(5.2) | 183(4.4) | 198(5.3) | 60(4.4) | 31(4.9) | 11(6.3) | 47(5.9) | |||
| No | 3987(95.5) | 3516(94.8) | 3969(95.6) | 3534(94.7) | 1,292(95.6) | 600(95.1) | 165(93.7) | 756(94.1) | |||
Summary of participants’ characteristics in baseline (2011) and follow-up study (2011–2018) (China Health and Retirement Longitudinal Study, China, 2011, 2013, 2015, 2018).
Note: N (%) was used for categorical variables: categorical variables were compared using chi-square tests analysis.
The Effects of Indoor Fuel Types on Non-Neoplastic Digestive Diseases at Baseline
In the fully adjusted models, compared to individuals who used clean fuel in the baseline, solid cooking fuel users showed a significantly increased risk of non-neoplastic digestive system diseases (NNDSD) (adjusted Hazard Ratio [aHR]: 1.17; 95% confidence interval [95% CI]: 1.02, 1.34). Similar results were found between solid (vs. clean) heating fuel use and NNDSD (aHR: 1.21; 95% CI: 1.07, 1.38). In addition, compared with those who were using clean fuel for both cooking and heating, the risk of NNDSD was found to be higher only in participants both cooking and heating with solid fuel (aHR: 1.34; 95% CI: 1.15, 1.55) (Table 2).
TABLE 2
| Variables | Events (rate/per 1,000 person years) | aHR (95%CI) | |
|---|---|---|---|
| Cooking fuel | |||
| Clean | 510(18.62) | Reference | |
| Solid | 551(22.64) | Model 1 | 1.23(1.09, 1.39) |
| Model 2 | 1.21(1.07, 1.37) | ||
| Model 3 | 1.18(1.02, 1.33) | ||
| Heating fuel | |||
| Clean | 510(18.53) | Reference | |
| Solid | 551(22.77) | Model 1 | 1.22(1.08, 1.37) |
| Model 2 | 1.20(1.06, 1.35) | ||
| Model 3 | 1.18(1.03, 1.37) | ||
| Cooking and heating fuel | |||
| Both clean for cooking and heating | 362(17.97) | Reference | |
| Clean for cooking and solid for heating | 148(20.04) | Model 1 | 1.12(0.92, 1.35) |
| Model 2 | 1.11(0.91, 1.34) | ||
| Model 3 | 1.10(0.92, 1.35) | ||
| Clean for heating and solid for cooking | 148(20.44) | Model 1 | 1.14(0.94, 1.38) |
| Model 2 | 1.12(0.92, 1.36) | ||
| Model 3 | 1.11(0.90, 1.39) | ||
| Both solid for cooking and heating | 403(23.77) | Model 1 | 1.33(1.15, 1.53) |
| Model 2 | 1.30(1.12, 1.50) | ||
| Model 3 | 1.31(1.12, 1.53) | ||
The associations between baseline household fuel exposure and NNDSD diagnosed during follow-up using Cox models (China Health and Retirement Longitudinal Study, China, 2011, 2013, 2015, 2018).
Model 1 is a crude model; Model 2 adjusts for age, sex, and educational level; Model 3 adjusts for age, sex, educational level, marital status, residence region, and smoking status. Rate: incidence rate per 1,000 person-years of follow-up, equal to (number of NNDSD events)/(person-years) 1000.
The Association of Cooking Fuel Type Conversion With Non-Neoplastic Digestive Diseases
In the fully adjusted model of the follow-up analysis, we found that those who with always solid cooking fuel had a higher risk (aHR: 1.42; 95% CI: 1.09, 1.84) of NNDSD compared to those with always clean cooking fuel; and those who switched from solid fuel to clean fuel had a lower risk (aHR: 0.65; 95% CI: 0.49, 0.87) of NNDSD compared to those with always solid cooking fuel (Table 3).
TABLE 3
| Variables | Events(rate) | aHR (95%CI) | |
|---|---|---|---|
| Consistent fuel type | |||
| Always clean fuel | 159(17.81) | Reference | |
| Always solid fuel | 137(26.56) | Model 1 | 1.50 (1.19, 1.88) |
| Model 2 | 1.47 (1.16, 1.86) | ||
| Model 3 | 1.42 (1.09, 1.84) | ||
| Had switched fuel type | |||
| Always solid fuel | 137(26.56) | Reference | |
| Solid to clean fuel | 73(17.22) | Model 1 | 0.65 (0.49, 0.86) |
| Model 2 | 0.65 (0.49, 0.87) | ||
| Model 3 | 0.65 (0.49, 0.87) | ||
The associations between fuel change patterns and NNDSD during follow-up using Cox models (China Health and Retirement Longitudinal Study, China, 2011, 2013, 2015, 2018).
Model 1 is a crude model; Model 2 adjusts for age, sex, and educational level; Model 3 adjusts for age, sex, educational level, marital status, residence region, and smoking status. Rate: incidence rate per 1,000 person-years of follow-up, equal to (number of NNDSD events)/(person-years) 1000.
Sensitivity Analyses
Males (aHR: 1.66; 95% CI: 1.16, 2.35), over 65 years participants (aHR: 2.63; 95% CI: 1.65, 4.21) and people without hypertension (aHR: 1.48; 95% CI: 1.12, 1.96) who consistently used solid fuels for cooking had a higher risk of NNDSD than those who continued to use clean fuels for cooking. Compared with those who consistently used solid fuels for cooking, participants aged 65+ (aHR: 0.44, 95% CI: 0.26, 0.73), those without hypertension (aHR: 0.65, 95% CI: 0.47, 0.90), men (aHR: 0.65, 95% CI: 0.43, 0.99) and women (aHR: 0.63, 95% CI: 0.42, 0.94) switching cooking fuels from solid to clean had significantly lower risk of NNDSD (Table 4).
TABLE 4
| Subgroups | Events (rate) | aHR(95%CI) |
|---|---|---|
| (A) | ||
| Always solid cooking fuel | ||
| Age(years) | ||
| 45–65 | 86 (24.61) | 1.18 (0.88, 1.57) |
| >65 | 51 (30.65) | 2.63 (1.65, 4.21) |
| Gender | ||
| Male | 71 (27.62) | 1.66 (1.16, 2.35) |
| Female | 66 (25.51) | 1.30 (0.93, 1.82) |
| Hypertension | ||
| Yes | 31 (28.65) | 1.52 (0.93, 2.49) |
| No | 106 (26.01) | 1.48 (1.12, 1.96) |
| (B) | ||
| Solid to clean cooking fuel | ||
| Age(years) | ||
| 45–65 | 49 (17.79) | 0.74 (0.52, 1.05) |
| >65 | 24 (16.16) | 0.44 (0.26, 0.73) |
| Gender | ||
| Male | 35 (18.60) | 0.65 (0.43, 0.99) |
| Female | 38 (16.12) | 0.63 (0.42, 0.94) |
| Hypertension | ||
| Yes | 18 (18.35) | 0.59 (0.32, 1.09) |
| No | 14 (4.30) | 0.65 (0.47, 0.90) |
The subgroup analyses the association between cooking fuel use and NNDSD from 2011 to 2018 (China Health and Retirement Longitudinal Study, China, 2011, 2013, 2015, 2018).
The multivariable-adjusted model adjusts for age, sex, educational level, marital status, residence region, and smoking status. Rate: incidence rate per 1,000 person-years of follow-up, equal to (number of NNDSD events)/(person-years) *1000. (A) The reference group is consistently clean cooking fuel. (B) The reference group is always solid cooking fuel.
The subgroup analyses stratified by baseline characteristics showed that males (aHR: 1.50; 95%CI: 1.21, 1.87), people aged 45–65 years (aHR: 1.37; 95%CI: 1.16, 1.63), and subjects without hypertension history (aHR: 1.35; 95%CI: 1.14, 1.60) using solid fuel for both cooking and heating had a significantly greater risk of NNDSD than those with clean cooking and heating fuels (Supplementary Figure S2). Similar results to the primary analyses were observed in subgroup analyses stratified by comorbidities, restricted to non-smokers, and additionally adjusting for population weighting concentrations of PM2.5 () and geographical weighting concentrations of PM2.5 (Supplementary Tables S2, S3, S5).
Discussion
Using the data from a large population-based cohort study, we observed that exposure to indoor air pollution (IAP) for heating and/or cooking was positively associated with non-neoplastic digestive system diseases (NNDSD) in both the baseline and the follow-up analyses. In addition, after switching fuel types from solid to clean, the risk of NNDSD was significantly lower than those with always solid fuel.
Several epidemiological types of research have reported evidence across countries and populations on the relationship between IAP and a wide range of adverse health events, including cardiovascular events [17], cardiopulmonary mortality [18], active tuberculosis [19], cervical cancer [20], incident arthritis [21], cognitive impairment [22]. Besides, limited empirical evidence on the relationship of air pollution with digestive diseases has been proposed [10, 12, 13]. An elderly population-based study from Hong Kong showed that short-term elevations in ambient nitrogen dioxide may increase the risk of bleeding peptic ulcers and consequent emergency hospital admissions [13]. An Italian study found that air pollution was associated with increased emergency room visits of gastroenteric disorder in children aged 0–2 years [10]. A multicity case-crossover study suggested that exposure to O3 frequently may increase the risk of perforated appendicitis [12]. Empirical evidence on the association of air pollution with the digestive disease remains preliminary, as the type of study design, short-term exposure to pollutants, or limitations in the study population that do not allow long-term follow-up analyses of exposure and outcomes; besides, the case-crossover study might be prone to information bias [10, 12]. However, less is known about the relationship between IAP (mainly caused by indoor solid fuel use) and digestive system diseases. The present large population-based cohort study first revealed an increased risk of NNDSD in individuals with long-term cooking and/or heating solid energy. Moreover, we also evaluated the effect of IAP change on NNDSD, which has rarely been considered in previous studies [10, 12, 13]. Our results partly provide evidence of the association between IAP and NNDSD.
A Longitudinal Healthy Longevity Survey in rural China reported that switching cooking fuel types crucially influenced the health effects and that switching from solid to clean fuel for cooking showed a significantly lower risk of decreased kidney function compared to consistently use of solid cooking fuel [23]. In the present study, we found that individuals who switched from solid to clean fuels for cooking had a significantly lower risk of NNDSD than those who continued to use solid fuels for cooking. More specifically, those aged more than 65 years, non-smokers, and without hypertension comorbidity had a substantially lower risk of NNDSD. This transformation in the type of cooking fuel is in line with the government’s three-year action plan (2013–2015) to address electricity use in non-electrified areas, which will promote the use of clean energy in part [24]. Cooking fuel type conversion is a modifiable behavior suggesting that increased financial support may promote clean fuel use and digestive disease prevention.
Several hypotheses underlying the association of air pollution and digestive system disorders have been proposed [1]: The pollutants can impair digestive system function, i.e., increasing intestinal permeability, altering the gut microbiome, and even increasing the risk of digestive organ cancers [13, 25, 26]. [2] Inhalation of higher concentrations of particulate matter can alter the metabolic level and stress hormones [27]. [3] Accumulating the detrimental effect of inhaled pollutants on lung inflammation and oxidative stress may contribute to systemic inflammation and oxidative stress [28, 29]. An animal study found significantly elevated mRNA expression of TNF-α in colon samples of mice exposed to particulate matter, suggesting that changes in the microbiota may induce gastrointestinal inflammation [24]. [30] The specific ambient pollutants may directly reach and interact with digestive organs [31, 32]. Overall, there is no systematic mechanism explaining the disturbance of the digestive system caused by solid fuel combustion, and further research is needed.
Previous studies about the impact of air pollution on sex have yielded heterogeneous results [33–35]. Most investigations have revealed that women are more likely to be damaged by air pollution, but some studies have reported the opposite [33–35]. In the baseline analysis, we observed a more pronounced effect of solid fuel on NNDSD in males than females. Smoking is widely recognized as another major cause of IAP(36), which produces many air pollutants, some of which are the same as those produced by solid fuel combustion and are hazardous to many human systems [36]. Published studies have documented smoking was associated with digestive tract diseases, including peptic ulcers [37], and ulcerative colitis [38]. The smoking prevalence of Chinese men is substantially higher than that of women [39]; lung function has been already impaired and may not recover within the short term, and may even have a synergistic effect with air pollution, increasing the risk of non-neoplastic digestive system diseases [40]. This may explain the higher estimate of solid fuel effect among males.
Our follow-up subgroup analyses with the data of 2011–2018 also found that people over 65 years exposed to cooking solid fuel had a higher risk of developing digestive problems. A time-series analysis in Nanjing of China provided suggestive evidence that older adults exposed to ambient air pollution are more likely to develop digestive illnesses [14]. Several studies have observed that elderly people are more vulnerable to indoor air pollution damage [41, 42]. Moreover, the elderly in China prefer to use solid living energy than youngers because of its low price [41, 43], increasing exposure to cooking solid fuel. These two reasons may explain why the estimated effect of cooking solid fuel is more pronounced in older groups.
The major strengths of the present study are as follows: the CHARLS adopts a multi-stage stratified probability-proportional-to-size sampling method in both the county/district and village sampling stages. The response rate and data quality of CHARLS rank at the top among similar projects in the world. Professionally trained investigators and staff guarantee the quality of the data collected by CHARLS.
Limitations
Several limitations should be noted. First, the use of self-reported diagnosis of NNDSD might have underestimated its prevalence, particularly among the older population and those with lower socioeconomic and educational backgrounds. Second, we obtained data on indoor fuel use by questionnaires, not by accurate external and internal exposure measurements. Moreover, we were unable to obtain data on concentrations of environmental air pollution to rule out potential impacts of specific pollutants on the digestive system. However, using measurements of global surface PM2.5 concentrations from the Atmospheric Composition Analysis Group at the University of Washington, we obtained the annual average PM2.5 concentration for each province of China [44]. We conducted baseline analysis of effect of solid fuel use for cooking or/and heating on NNDSD additionally adjusted for PM2.5. The results of the analysis after the inclusion of external confounding are similar to those of the main analysis, which indicates the stability of our results. Future studies might include objective assessment of external pollutants at the individual level. Third, NNDSD in the present study covers all non-neoplastic digestive system diseases, and there is no information about NNDSD subtypes (Peptic ulcer, inflammatory bowel disease, etc.); further studies may explore such associations in different NNDSD subtypes. Finally, the information on heating fuel during follow-up is unavailable. Future studies could incorporate heating fuel usage to fully explore the impact of fuel type conversion on adverse outcomes.
Conclusion
Indoor solid fuel use for cooking and/or heating was an independent risk factor for non-neoplastic digestive system diseases (NNDSD). Additionally, switching fuel types from solid to clean cooking fuels may help reduce the impact of indoor solid fuel use on NNDSD. The present study highlights the urgency of switching to clean cooking fuels, with major public health implications for developing countries.
Statements
Data availability statement
The datasets generated and analyzed during the current study are not publicly available due to separate ongoing original analyses being performed but are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review board of Peking University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
GQ and JC take responsibility for the accuracy of the study design and data analysis. YL conducted data extraction and statistical analyses. YL wrote the first draft of the manuscript. All authors revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (grants 82173612); the National Natural Science Foundation of China (grants 82273730); Shanghai Rising-Star Program (21QA1401300); Shanghai Municipal Natural Science Foundation (22ZR1414900); National Key R&D Program of China (2021YFC2502200); Shanghai Municipal Science and Technology Major Project (ZD2021CY001).
Acknowledgments
The authors thank all the staff and the participants in the project.
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2022.1605419/full#supplementary-material
References
1.,
GBD 2019 Risk Factors Collaborators. Global burden of 87 Risk Factors in 204 Countries and Territories, 1990-2019: a Systematic Analysis for the Global Burden of Disease Study 2019. Lancet (2020) 396(10258):1223–49. 10.1016/S0140-6736(20)30752-2
2.
Lee KK Bing R Kiang J Bashir S Spath N Stelzle D et al Adverse Health Effects Associated with Household Air Pollution: a Systematic Review, Meta-Analysis, and burden Estimation Study. Lancet Glob Health (2020) 8(11):e1427–34. 10.1016/S2214-109X(20)30343-0
3.
Smith KR Bruce N Balakrishnan K Adair-Rohani H Balmes J Chafe Z et al Millions Dead: How Do We Know and what Does it Mean? Methods Used in the Comparative Risk Assessment of Household Air Pollution. Annu Rev Public Health (2014) 35:185–206. 10.1146/annurev-publhealth-032013-182356
4.
Health Effects Institute. State of Global Air 2019. Boston, MA: Health Effects Institute (2019).
5.
Ng SC Shi HY Hamidi N Underwood FE Tang W Benchimol EI et al Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st century: a Systematic Review of Population-Based Studies. Lancet (2017) 390(10114):2769–78. 10.1016/S0140-6736(17)32448-0
6.
Kaplan GG Ng SC . Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology (2017) 152(2):313–21. 10.1053/j.gastro.2016.10.020
7.
Forman D Newell DG Fullerton F Yarnell JW Stacey AR Wald N et al Association between Infection with Helicobacter pylori and Risk of Gastric Cancer: Evidence from a Prospective Investigation. BMJ (1991) 302(6788):1302–5. 10.1136/bmj.302.6788.1302
8.
Parsons BN Ijaz UZ D'Amore R Burkitt MD Eccles R Lenzi L et al Comparison of the Human Gastric Microbiota in Hypochlorhydric States Arising as a Result of Helicobacter Pylori-Induced Atrophic Gastritis, Autoimmune Atrophic Gastritis and Proton Pump Inhibitor Use. Plos Pathog (2017) 13(11):e1006653. 10.1371/journal.ppat.1006653
9.
Uemura N Okamoto S Yamamoto S Matsumura N Yamaguchi S Yamakido M et al Helicobacter pylori Infection and the Development of Gastric Cancer. N Engl J Med (2001) 345(11):784–9. 10.1056/NEJMoa001999
10.
Orazzo F Nespoli L Ito K Tassinari D Giardina D Funis M et al Air Pollution, Aeroallergens, and Emergency Room Visits for Acute Respiratory Diseases and Gastroenteric Disorders Among Young Children in Six Italian Cities. Environ Health Perspect (2009) 117(11):1780–5. 10.1289/ehp.0900599
11.
Gu J Shi Y Zhu Y Chen N Wang H Zhang Z et al Ambient Air Pollution and Cause-specific Risk of Hospital Admission in China: A Nationwide Time-Series Study. Plos Med (2020) 17(8):e1003188. 10.1371/journal.pmed.1003188
12.
Kaplan GG Tanyingoh D Dixon E Johnson M Wheeler AJ Myers RP et al Ambient Ozone Concentrations and the Risk of Perforated and Nonperforated Appendicitis: a Multicity Case-Crossover Study. Environ Health Perspect (2013) 121(8):939–43. 10.1289/ehp.1206085
13.
Tian L Qiu H Sun S Tsang H Chan KP Leung WK . Association between Emergency Admission for Peptic Ulcer Bleeding and Air Pollution: a Case-Crossover Analysis in Hong Kong's Elderly Population. Lancet Planet Health (2017) 1(2):e74–e81. 10.1016/S2542-5196(17)30021-9
14.
Wang C Zhu G Zhang L Chen K . Particulate Matter Pollution and Hospital Outpatient Visits for Endocrine, Digestive, Urological, and Dermatological Diseases in Nanjing, China. Environ Pollut (2020) 261:114205. 10.1016/j.envpol.2020.114205
15.
Zhao Y Hu Y Smith JP Strauss J Yang G . Cohort Profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol (2014) 43(1):61–8. 10.1093/ije/dys203
16.
Lin DY Wei L-J Ying Z . Checking the Cox Model with Cumulative Sums of Martingale-Based Residuals. Biometrika (1993) 80(3):557–72. 10.1093/biomet/80.3.557
17.
Huang S Guo C Qie R Han M Wu X Zhang Y et al Solid Fuel Use and Cardiovascular Events: A Systematic Review and Meta-Analysis of Observational Studies. Indoor Air (2021) 31(6):1722–32. 10.1111/ina.12867
18.
Yu K Lv J Qiu G Yu C Guo Y Bian Z et al Cooking Fuels and Risk of All-Cause and Cardiopulmonary Mortality in Urban China: a Prospective Cohort Study. Lancet Glob Health (2020) 8(3):e430–9. 10.1016/S2214-109X(19)30525-X
19.
Dorjravdan M Kouda K Boldoo T Dambaa N Sovd T Nakama C et al Association between Household Solid Fuel Use and Tuberculosis: Cross-Sectional Data from the Mongolian National Tuberculosis Prevalence Survey. Environ Health Prev Med (2021) 26(1):76. 10.1186/s12199-021-00996-4
20.
Liu T Song Y Chen R Zheng R Wang S Li L . Solid Fuel Use for Heating and Risks of Breast and Cervical Cancer Mortality in China. Environ Res (2020) 186:109578. 10.1016/j.envres.2020.109578
21.
Deng Y Gao Q Yang T Wu B Liu Y Liu R . Indoor Solid Fuel Use and Incident Arthritis Among Middle-Aged and Older Adults in Rural China: A Nationwide Population-Based Cohort Study. Sci Total Environ (2021) 772:145395. 10.1016/j.scitotenv.2021.145395
22.
Cao L Zhao Z Ji C Xia Y . Association between Solid Fuel Use and Cognitive Impairment: A Cross-Sectional and Follow-Up Study in a Middle-Aged and Older Chinese Population. Environ Int (2021) 146:106251. 10.1016/j.envint.2020.106251
23.
Xue B Wang B Lei R Li Y Luo B Yang A et al Indoor Solid Fuel Use and Renal Function Among Middle-Aged and Older Adults: A National Study in Rural China. Environ Res (2022) 206:112588. 10.1016/j.envres.2021.112588
24.
Tang X Liao H . Energy Poverty and Solid Fuels Use in Rural China: Analysis Based on National Population Census. Energ Sustain Dev (2014) 23:122–9. 10.1016/j.esd.2014.08.006
25.
Opstelten JL Beelen RMJ Leenders M Hoek G Brunekreef B van Schaik FDM et al Exposure to Ambient Air Pollution and the Risk of Inflammatory Bowel Disease: A European Nested Case-Control Study. Dig Dis Sci (2016) 61(10):2963–71. 10.1007/s10620-016-4249-4
26.
Alderete TL Jones RB Chen Z Kim JS Habre R Lurmann F et al Exposure to Traffic-Related Air Pollution and the Composition of the Gut Microbiota in Overweight and Obese Adolescents. Environ Res (2018) 161:472–8. 10.1016/j.envres.2017.11.046
27.
Li H Cai J Chen R Zhao Z Ying Z Wang L et al Particulate Matter Exposure and Stress Hormone Levels: A Randomized, Double-Blind, Crossover Trial of Air Purification. Circulation (2017) 136(7):618–27. 10.1161/CIRCULATIONAHA.116.026796
28.
Chin MT . Basic Mechanisms for Adverse Cardiovascular Events Associated with Air Pollution. Heart (2015) 101(4):253–6. 10.1136/heartjnl-2014-306379
29.
Rajagopalan S Al-Kindi SG Brook RD . Air Pollution and Cardiovascular Disease: JACC State-Of-The-Art Review. J Am Coll Cardiol (2018) 72(17):2054–70. 10.1016/j.jacc.2018.07.099
30.
Balmes JR . Household Air Pollution from Domestic Combustion of Solid Fuels and Health. J Allergy Clin Immunol (2019) 143(6):1979–87. 10.1016/j.jaci.2019.04.016
31.
Miller MR Raftis JB Langrish JP McLean SG Samutrtai P Connell SP et al Inhaled Nanoparticles Accumulate at Sites of Vascular Disease. ACS Nano (2017) 11(5):4542–52. 10.1021/acsnano.6b08551
32.
Maher BA Ahmed IA Karloukovski V MacLaren DA Foulds PG Allsop D et al Magnetite Pollution Nanoparticles in the Human Brain. Proc Natl Acad Sci U S A (2016) 113(39):10797–801. 10.1073/pnas.1605941113
33.
Kuźma Ł Struniawski K Pogorzelski S Bachorzewska-Gajewska H Dobrzycki S . Gender Differences in Association between Air Pollution and Daily Mortality in the Capital of the Green Lungs of Poland-Population-Based Study with 2, 953, 000 Person-Years of Follow-Up. J Clin Med (2020) 9(8):2351. 10.3390/jcm9082351
34.
Wang N Mengersen K Tong S Kimlin M Zhou M Wang L et al Short-term Association between Ambient Air Pollution and Lung Cancer Mortality. Environ Res (2019) 179:108748. 10.1016/j.envres.2019.108748
35.
Ma Y Zhao Y Yang S Zhou J Xin J Wang S et al Short-term Effects of Ambient Air Pollution on Emergency Room Admissions Due to Cardiovascular Causes in Beijing, China. Environ Pollut (2017) 230:974–80. 10.1016/j.envpol.2017.06.104
36.
Talhout R Schulz T Florek E van Benthem J Wester P Opperhuizen A . Hazardous Compounds in Tobacco Smoke. Int J Environ Res Public Health (2011) 8(2):613–28. 10.3390/ijerph8020613
37.
Aro P Ronkainen J Storskrubb T Vieth M Engstrand L Johansson SE et al Use of Tobacco Products and Gastrointestinal Morbidity: an Endoscopic Population-Based Study (The Kalixanda Study). Eur J Epidemiol (2010) 25(10):741–50. 10.1007/s10654-010-9495-8
38.
Mahid SS Minor KS Soto RE Hornung CA Galandiuk S . Smoking and Inflammatory Bowel Disease: a Meta-Analysis. Mayo Clin Proc (2006) 81(11):1462–71. 10.4065/81.11.1462
39.
Wang M Luo X Xu S Liu W Ding F Zhang X et al Trends in Smoking Prevalence and Implication for Chronic Diseases in China: Serial National Cross-Sectional Surveys from 2003 to 2013. Lancet Respir Med (2019) 7(1):35–45. 10.1016/S2213-2600(18)30432-6
40.
Willemse BW Postma DS Timens W ten Hacken NH . The Impact of Smoking Cessation on Respiratory Symptoms, Lung Function, Airway Hyperresponsiveness and Inflammation. Eur Respir J (2004) 23(3):464–76. 10.1183/09031936.04.00012704
41.
Hou B Liao H Wang JW Wang F Zhang H . Cooking Fuel Decision-Making and Family Structure: a Field Study in China. Environ Sci Pollut Res Int (2019) 26(23):24050–61. 10.1007/s11356-019-05216-9
42.
Tsabouri S Bleta AG Nastos PT Priftis KN . Ambient Environmental Risk Factors for Childhood Wheezing Illness. Front Biosci (Elite Ed (2015) 7(3):447–68. 10.2741/E742
43.
Gheewala SH Damen B Shi X . Biofuels: Economic, Environmental and Social Benefits and Costs for Developing Countries in Asia. Wiley Interdiscip Rev Clim Change (2013) 4(6):497–511. 10.1002/wcc.241
44.
van Donkelaar A Hammer MS Bindle L Brauer M Brook JR Garay MJ et al Monthly Global Estimates of Fine Particulate Matter and Their Uncertainty. Environ Sci Technol (2021) 55(22):15287–300. 10.1021/acs.est.1c05309
Summary
Keywords
solid fuels, household air pollution, clean fuels, digestive diseases, middle-aged and older adults
Citation
Liu Y, Zeng S, Huang C, Wang C, Zhu J, Peng J, Ding F, Li J, Qin G and Chen J (2022) Indoor Solid Fuel Use and Non-Neoplastic Digestive System Diseases: A Population-Based Cohort Study Among Chinese Middle-Aged and Older Population. Int J Public Health 67:1605419. doi: 10.3389/ijph.2022.1605419
Received
20 September 2022
Accepted
12 December 2022
Published
21 December 2022
Volume
67 - 2022
Edited by
Kalpana Balakrishnan, Sri Ramachandra University, India
Reviewed by
Santu Ghosh, St John’s Medical College, India
Jian Hou, Zhengzhou University, China
Updates
Copyright
© 2022 Liu, Zeng, Huang, Wang, Zhu, Peng, Ding, Li, Qin and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaohua Chen, jiairchen@126.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.