Abstract
Objectives: This systematic review and meta-analysis aimed to: i) determine the pooled prevalence of acute diarrhea; and ii) synthesize and summarize current evidence on factors of acute diarrheal illnesses among under-five children in Ethiopia.
Methods: A comprehensive systematic search was conducted in PubMed, SCOPUS, HINARI, Science Direct, Google Scholar, Global Index Medicus, Directory of Open Access Journals (DOAJ), and the Cochrane Library. This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. The methodological quality of each included article was assessed using the Joanna Briggs Institute (JBI) quality assessment tool for cross-sectional and case-control studies. A random-effect meta-analysis model was used to estimate the pooled prevalence of diarrheal illnesses. Heterogeneity and publication bias were assessed using I2 test statistics and Egger’s test, respectively. The statistical analysis was done using STATA™ software version 14.
Results: Fifty-three studies covering over 27,458 under-five children who met the inclusion criteria were included. The pooled prevalence of diarrhea among under-five children in Ethiopia was found to be 20.8% (95% CI: 18.69–22.84, n = 44, I2 = 94.9%, p < 0.001). Our analysis revealed a higher prevalence of childhood diarrhea in age groups of 12–23 months 25.42% (95%CI: 21.50–29.35, I2 = 89.4%, p < 0.001). In general, the evidence suggests that diarrheal risk factors could include: i) child level determinants (child’s age 0–23 months, not being vaccinated against rotavirus, lack of exclusive breastfeeding, and being an under-nourished child); ii) parental level determinants {mothers poor handwashing practices [pooled odds ratio (OR) = 3.05; 95% CI:2.08–4.54] and a history of maternal recent diarrhea (pooled OR = 3.19, 95%CI: 1.94–5.25)}; and iii) Water, Sanitation and Hygiene (WASH) determinants [lack of toilet facility (pooled OR = 1.56, 95%CI: 1.05–2.33)], lack handwashing facility (pooled OR = 4.16, 95%CI: 2.49–6.95) and not treating drinking water (pooled OR = 2.28, 95% CI: 1.50–3.46).
Conclusion: In Ethiopia, the prevalence of diarrhea among children under the age of five remains high and is still a public health problem. The contributing factors to acute diarrheal illnesses were child, parental, and WASH factors. A continued focus on improving access to WASH facilities, along with enhancing maternal hygiene behavior will accelerate reductions in diarrheal disease burden in Ethiopia.
Introduction
Childhood diarrheal disease remains to be the existential threat to global public health scourge. According to the Global Burden of Disease Study 2019, diarrhea disease was listed among the top three most common problems causing a significant health burden in children [1]. Evidence indicates that deaths from diarrheal disease among children under 5 are most prevalent in South Asia and sub-Saharan Africa, where access to healthcare, safe water, and sanitation remains limited [2, 3]. In Ethiopia, although there has been a reduction in the prevalence of diarrheal disease from 24% in 2000 to 12% in 2016, progress has not been sufficiently rapid to fully tackle this significant public health issue [4, 5].
Previous studies conducted in Ethiopia have identified many factors that are associated with childhood diarrhea. These factors include: child’s age [6–10], place of residency [11–13], lack of exclusive breastfeeding [14–17], unvaccinated against rotavirus [7, 14, 18–22], undernutrition [23–25], limited maternal education [11, 23, 24, 26], inadequate knowledge about diarrheal disease [8, 27], poor handwashing practices [15, 24, 28, 29], low wealth status [14, 26, 27, 30, 31], unimproved sources of drinking water [6, 9, 12, 13, 19, 32], and unimproved toilet facilities [7, 19, 20, 32–36].
Despite the fact that diarrhea is largely preventable, it remains a public health problem in Ethiopia, and its burden is still a serious concern. Estimates of the burden of diarrheal disease and its associated factors in Ethiopia at the national level are not well known, masking the current status, a prohibitive factor to tracking progress and reducing morbidity. While it is known this to be an ongoing and significant public health issue needing current evidence, the most review evidence have been over 5 years old [37], which may not reflect the present situation. Additionally, preliminary studies that have recently been conducted throughout the country have reported inconsistent findings [7, 12, 13, 26, 31, 33, 34, 38–44], making it necessary to update the existing review in order to estimate the current nationwide and regional pooled prevalence of diarrheal diseases and associated factors. Therefore, this systematic review and meta-analysis provide updated results of pooled estimates of childhood diarrhea morbidity and summarized its associated factors in Ethiopia. The findings affirm the current status of diarrheal diseases and set a benchmark for tracking the progress toward achieving the Sustainable Development Goals (SDG).
Methods
Protocol Registry
The protocol for this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO), the University of York Centre for Reviews and Dissemination (record ID: CRD42022354416).
Search Strategy and Information Sources
This is an update of the systematic review and meta-analysis, which was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines 2020 [45] (Supplementary Material S1).
Eligibility Criteria
Population: children under the age of 5 years (under-fives).
The outcome of interest: prevalence of acute diarrheal illness. Studies that reported diarrhea above 2 weeks were excluded.
Study design: Observational studies (cohort, case-control, and cross-sectional studies) that assessed the prevalence and associated factors of under-five diarrheal disease were included in the study. Any observational studies that did not report either the prevalence or associated factors of acute diarrhea by controlling possible confounders were excluded from the study. Additionally, interventional studies (randomized controlled trials (RCTs), cluster randomized controlled trials (cRCTs) and quasi-experimental (QE) trials), systematic reviews, commentaries, letters to editors, qualitative studies, case studies, books, reports, conference abstracts, non-primary studies, and analysis of policy briefs were excluded.
Study setting: Only studies conducted in Ethiopia.
Publication status: Both published and unpublished studies were considered.
Language: Articles published in the English language were considered.
Publication year: All articles published since 2017 were considered.
Operational Definition
Acute diarrhea: is defined as the passage of three or more loose or liquid stools per day or an increase in stool frequency or liquidity that is considered abnormal during the 2 weeks as reported by the mothers or caregivers [46].
Information Sources
A comprehensive review of English language literature using list of relevant Medical Subject Heading Terms (MeSH) words and sub-headings of keywords was generated and used to search articles from major databases including PubMed, SCOPUS, HINARY, Science Direct, Google Scholar, Global Index Medicus, Directory of Open Access Journals (DOAJ), and the Cochrane Library. Each database’s or search engine’s retrieved articles were added to an EndNote library. Additionally, articles from the retrieved bibliographies that met the inclusion criteria were added to be reviewed. We also used Google Scholar and Google to track citations.
Searching Strategy
The following combination of keywords and other MeSH words was used in the search: diarrhea [MeSH Terms], Diarrhea [Text Word], Diarrhoea [All Filed], Prevalence [Text Word], Factor*, determinant*, child [MeSH Terms], infant [MeSH Terms], under-five children, and Ethiopia. The search terms were used separately and in combination using Boolean operators like “OR” or “AND.” A detail searching strategy was provide in the Supplementary Material S2. The electronic database search was supplemented with gray literature searches via Google Scholar and Google searches. A secondary search method known as “footnote chasing” was also used to identify further and relevant articles. Reference lists of included studies were manually searched for additional relevant articles. Moreover, preprint articles from the MedRxiv, BioRxiv, and Research square databases were also accessed to ensure wider coverage. The related systematic review and meta-analysis published in 2017, in which the subjects from the included studies were mostly enrolled before 2017. In this review, all papers published from first of November 2017 to June 30th, 2022, were considered. All papers published until the June 30th, 2022 were considered. An initial search was conducted on June 1st, 2022 and later repeated on June 30th, 2022 to identify articles.
Study Selection Process
In accordance with predetermined inclusion and exclusion criteria, two investigators (BS and DA) independently screened and identified relevant articles by title, abstract, and full text. The screened articles were compiled by the two authors (BS and DA), and any disagreements between them were settled through discussion. Duplicate articles were then removed from the review after all the searched articles were exported into the EndNote™ version X8 software. After reading the complete texts of the remaining articles, we retained studies that met the inclusion criteria. Based on the eligibility requirements, BS and DA independently reviewed the full text of the articles based on the study eligibility criteria.
Data Extraction
Microsoft™ Excel was used to extract the data. The primary author, publication year, study design, study area, sample size, prevalence of diarrhea, number of children, age of children, sampling techniques, and factors associated with diarrhea were included in the summary of the studies that were included in the review.
Risk of Bias Assessment of the Studies
The quality of the included studies was evaluated using the Joanna Briggs Institute (JBI) method for quality evaluation. We used the JBI checklist for case-control studies and observational studies were used [47]. Two reviewers independently evaluated the included studies’ quality (BS and DA). There are eight and ten parameters in the evaluation tools for cross-sectional and case-control studies, respectively. When the information provided was insufficient to make a decision, we decided to assess a 1 rating for the specific item (a failure to satisfy a specific item or unclear/not applicable). Bias risks were divided into three categories: low (0–2), moderate (3 or 4), and high (total score of 5 or higher). Disagreements are usually remedied through discussion until consensus is reached. However, for the current study, there was no disagreement in selecting the potential research (Supplementary Material S3).
Synthesis of Results
The prevalence of diarrhea and/or associated factors were used in reporting the findings of each study. Where available, 95% CI for reported diarrhea was obtained from the eligible studies. Based on published studies, 95% CI were calculated using available data where the full text of the eligible studies did not report diarrheal estimates. Narrative methods including text and table were used as a tool for associated factors data presentation. The heterogeneity among included studies was assessed by I2 statistics and the Cochran Q-test. The included studies exhibited significant high heterogeneity (I2 = 94.9%, p < 0.001), which led us to compute a random effect meta-analysis model to estimate the pooled prevalence of diarrhea. The pooled prevalence of diarrhea and their corresponding 95% CI were presented using a forest plot. Subgroup analyses were performed to investigate the observed heterogeneity, based on the sub-regions of Ethiopia. Further statistical analyses, such as univariate meta-regression, was also performed to identify possible sources of heterogeneity.
We used a qualitative approach to summarize the factors associated with diarrhea and synthesize the relevant information based on the objectives of the study. We did not perform a meta-analysis for all identified factors due to the considerable heterogeneity of the included studies. We have discussed identified associated factors and estimates descriptively. For some variables, however, the adjusted odds ratios (AOR) were pooled using the generic inverse variance method, which involved converting the adjusted odds ratio to a logarithmic scale and then calculating standard error based on the 95% confidence intervals [48]. The Cochran Q-test and Haggin I2 statistics were used to assess the presence and degree of heterogeneity among included studies [49].
Publication Bias
In this meta-analysis, the presence of publication bias was evaluated using funnel plots and Egger’s weighted regression test at a significance level of less than 0.05.
Sensitivity Analysis
To identify the source of heterogeneity, a leave-one-out analysis was employed. Sensitivity analysis using a random-effects model was performed to assess the influence of a single study on the overall pooled prevalence estimate.
Results
Study Selection
Overall, the searches identified 707 articles (i.e., identification of studies via databases and registers). Of the initial articles, 444 articles were excluded due to duplication. We screened the titles and abstracts of 263 articles and obtained 91 full text articles, of which 53 studies met the inclusion criteria and were included in the final systematic review and meta-analysis. Of the included 53 studies, 44 studies were eligible for meta-analysis (Figure 1).
FIGURE 1
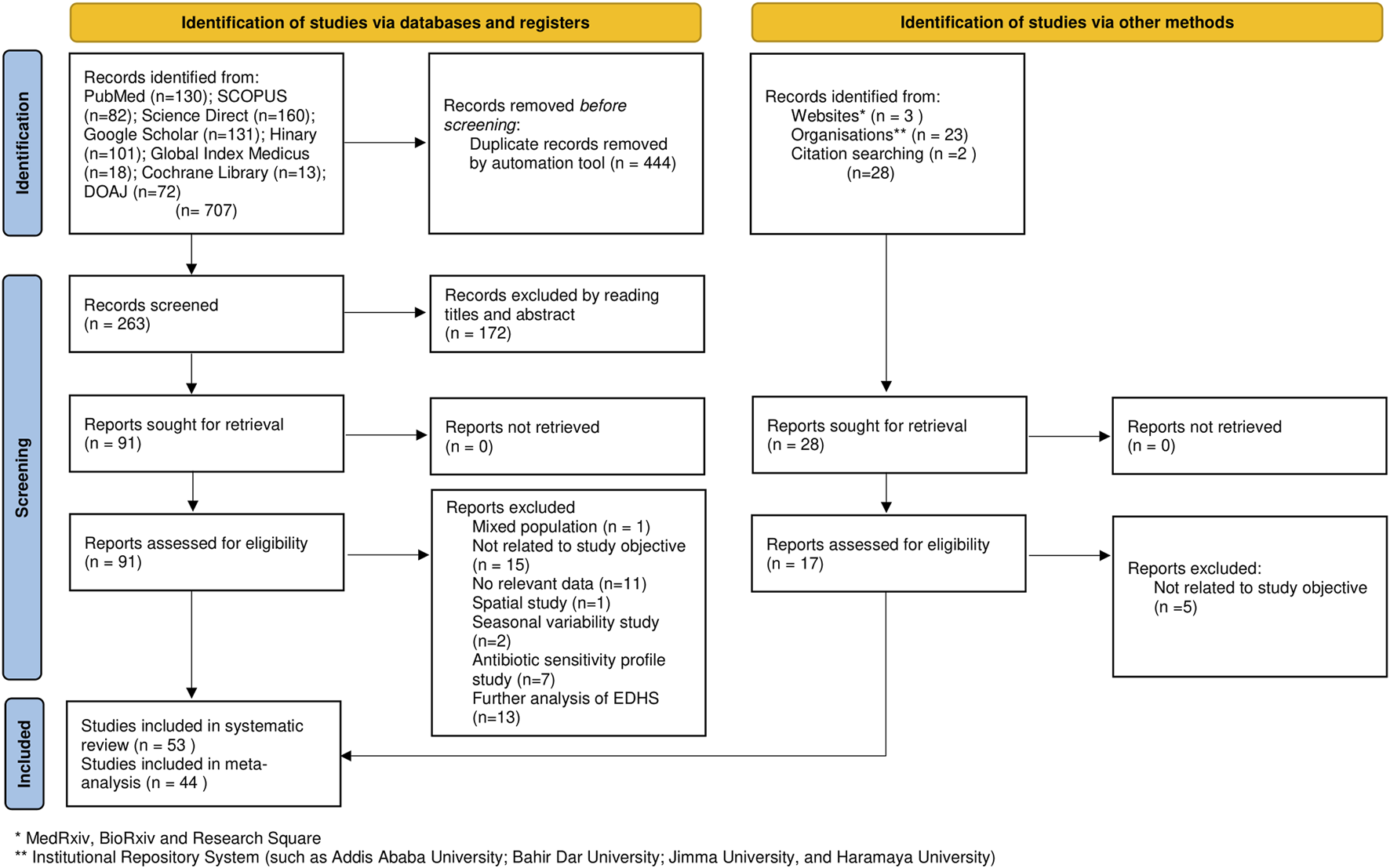
Flow chart of study selection for systematic review and meta-analysis of prevalence of acute diarrhea and associated factors among under-five children in Ethiopia, (Ethiopia, 2017–2022).
Characteristics of Included Studies
The descriptive summary of included cross-sectional and case-control studies were presented in Table 1. Of the included 53 studies, 9 were case-control studies, 12 comparative cross-sectional studies, and 32 cross-sectional studies by design. A total of 27,458 under-five children were included as participants in the current systematic review and meta-analysis. In the current review, the sample size varied from small (n = 191) [61] to large (n = 2,030) [6]. In this review, the lowest prevalence of diarrhea (9.4%) was found in a study conducted in Bahir Dar, northwest Ethiopia Amhara region [14], while the highest prevalence (36.5%) of diarrhea was reported in a study conducted at west Guji zone, Oromia region [62].
TABLE 1
| Author | Publication year | Sample size | Prevalence, 95%CI | Study design | Study population | Region | Sampling method | |
|---|---|---|---|---|---|---|---|---|
| 1 | Alemayehu M et al. [19] | 2020 | 722 | 23.5 (20.4–26.6) | CS | 0–59 months | SNNPR | Multistage sampling technique |
| 2 | Hailu B et al. [33] | 2021 | 419 | 25.3 (21.1–29.5) | CS | 0–4 years | Amhara | Systematic sampling technique |
| 3 | Tesfaye TS et al. [34] | 2020 | 311 | 30.9 (25.8–36.0) | CS | 0–59 months | SNNPR | Systematic sampling technique |
| 4 | Soboksa NE et al. [50] | 2021 | 756 | 19.8 (17.0–22.6) | CCS | 0–59 months | Oromia | Multistage sampling technique |
| 5 | Getahun W et al. [41] | 2021 | 484 | 17.6 (14.2–21.0) | CS | 0–59 months | Amhara | Systematic sampling technique |
| 6 | Dagnew AB et al. [38] | 2019 | 498 | 14.5 (11.4–17.6) | CS | 0–59 months | Amhara | Systematic sampling technique |
| 7 | Getachew A et al. [51] | 2018 | 736 | 22.1 (19.1–25.1) | CS | 0–59 months | Amhara | Systematic sampling technique |
| 8 | Gebrezgiabher BB et al. [35] | 2019 | 652 | 13.9 (11.2–16.6) | CCS | 0–59 months | Tigray | Multistage sampling technique |
| 9 | Wasihun AG et al. [42] | 2018 | 610 | 27.2 (23.7–30.7) | CS | 6–59 months | Tigray | Multistage stratified sampling |
| 10 | Ayalew AM et al. [52] | 2018 | 525 | 23.0 (19.4–26.6) | CCS | 0–59 months | Amhara | Multistage sampling technique |
| 11 | Adane M et al. [30] | 2018 | 690 | 11.9 (9.5–14.3) | CS | 0–50 months | Addis Ababa | Multistage sampling technique |
| 12 | Melese B et al. [24] | 2019 | 537 | 13.6 (10.7–16.5) | CS | 0–59 months | Sidama | Multistage sampling technique |
| 13 | Shumetie G et al. [14] | 2018 | 553 | 9.4 (7.0–11.8) | CS | 0–59 months | Amhara | Multistage sampling technique |
| 14 | Mekonnen GK et al. [36] | 2019 | 813 | 33 (29.8–36.2) | CCS | 0–59 months | Gambella | Stratified multistage sampling |
| 15 | Degebasa MZ et al. [53] | 2018 | 760 | 25 (21.9–28.1) | CCS | 0–59 months | Addis Ababa | Multistage sampling method |
| 16 | Mernie G et al. [40] | 2022 | 654 | 15.7 (12.9–18.5) | CCS | 0–59 months | Amhara | Multistage sampling technique |
| 17 | Wagari S et al. [54] | 2022 | 535 | 24.8 (21.1–28.5) | CS | 0–59 months | Oromia | Simple random sampling technique |
| 18 | Feleke Y et al. [15] | 2022 | 440 | 17.3 (13.8–20.8) | CS | 0–59 months | Oromia | Systematic sampling technique |
| 19 | Fufa WK et al. [55] | 2019 | 559 | 22 (18.6–25.4) | CCS | 6–59 months | Oromia | Multistage cluster sampling |
| 20 | Feleke DG et al. [56] | 2022 | 717 | 14.5 (11.9–17.1) | CS | 0–59 months | Amhara | Two stage multi-stage sampling |
| 21 | Megersa S et al. [57] | 2019 | 709 | 20.2 (17.2–23.2) | CCS | 0–59 months | Oromia | Two stage multi-stage sampling |
| 22 | Beyene SG et al. [58] | 2018 | 450 | 28.4 (24.2–32.6) | CS | 0–59 months | Oromia | Multistage sampling |
| 23 | Kasee LF et al. [59] | 2018 | 250 | 14.9 (10.5–19.3) | CS | 0–59 months | Oromia | Systematic sampling |
| 24 | Gashaw TA et al. [23] | 2019 | 582 | 21.8 (18.4–25.2) | CS | 0–59 months | SNNPR | Multi-stage sampling |
| 25 | Bitew BD et al. [31] | 2022 | 407 | 24.9 (20.7–29.1) | CS | 0–59 months | Amhara | Simple random sampling technique |
| 26 | Getachew F et al. [60] | 2022 | 450 | 13.6 (10.4–16.8) | CS | 0–59 months | Addis Ababa | Systematic sampling technique |
| 27 | Zedie FB et al. [28] | 2018 | 808 | 14.7 (12.3–17.1) | CCS | 0–59 months | Sidama | Multistage sampling technique |
| 28 | Chomissa AR et al. [20] | 2018 | 718 | 16.7 (14.0–19.4) | CCS | 0–59 months | SNNPR | Multistage sampling technique |
| 29 | Arba A et al. [89] | 2020 | 223 | 27.3 (21.5–33.1) | CS | 0–59 months | SNNPR | Systematic sampling |
| 30 | Amamo DD et al. [62] | 2019 | 717 | 36.5 (33.0–40.0) | CS | 0–59 months | Oromia | Multistage cluster random sampling |
| 31 | Zegeye Z [90] | 2021 | 554 | 26.4 (22.7–30.1) | CS | 0–59 months | Amhara | Multistage sampling technique |
| 32 | Mengistu KD [61] | 2021 | 191 | 25.7 (19.5–31.9) | CS | 0–59 months | Amhara | Simple random sampling technique |
| 33 | Angasu K et al. [6] | 2022 | 2030 | 34.5 (32.4–36.6) | CS | 0–59 months | SNNPR | Two-stage sampling procedure |
| 34 | Mitiku HD [13] | 2021 | 873 | 19.8 (17.2–22.4) | CS | 0–59 months | Amhara | Systematic sampling technique |
| 35 | Kassie G [91] | 2020 | 661 | 11 (8.6–13.4) | CS | 2–59 months | Amhara | Multistage sampling |
| 36 | Alemayehu B et al. [21] | 2020 | 826 | 18.3 (15.7–20.9) | CS | 0–59 months | SNNPR | Stratified sampling |
| 37 | Natnael T et al. [9] | 2021 | 335 | 11 (7.7–14.3) | CS | 0–59 months | Amhara | Systematic sampling |
| 38 | Bekele D et al. [22] | 2021 | 512 | 17.8 (14.5–21.1) | CCS | 0–59 months | Oromia | Multi-stage sampling technique |
| 39 | Fenta A et al. [43] | 2020 | 717 | 14.5 (11.9–17.1) | CS | 0–59 months | Beni Shangul Gumuz | Multistage sampling |
| 40 | Alemayehu K et al. [8] | 2021 | 620 | 24 (20.6–27.4) | CS | 0–59 months | Oromia | Simple random sampling |
| 41 | Shine S et al. [18] | 2020 | 420 | 16.4 (12.9–19.9) | CS | 0–59 months | Amhara | Multi-stage sampling |
| 42 | Solomon ET et al. [44] | 2020 | 1,146 | 23 (20.6–25.4) | CS | 0–59 months | Dire Dawa | Multi-stage sampling procedure |
| 43 | Tafere Y et al. [26] | 2020 | 758 | 29.9 (26.6–33.2) | CCS | 0–59 months | Amhara | Systematic sampling technique |
| 44 | Mulu E et al. [32] | 2022 | 530 | 21.3 (17.8–24.8) | CS | 0–59 months | SNNPR | Multi-stage sampling procedures |
| 45 | Brhanu H et al. [11] | 2017 | 618 | - | CC | 0–59 months | Benishangul Gumuz | Simple random sampling technique |
| 46 | Derseh BT et al. [27] | 2021 | 309 | - | CC | 6–59 months | Amhara | Systematic sampling technique |
| 47 | Delelegn MW et al. [39] | 2020 | 306 | - | CC | 0–59 months | Amhara | Systematic sampling technique |
| 48 | Mosisa D et al. [7] | 2021 | 399 | - | CC | 0–59 months | Oromia | Systematic sampling technique |
| 49 | Baye A et al. [16] | 2021 | 357 | - | CC | 0–23 months | Amhara | Simple random sampling technique |
| 50 | Soboksa NE et al. [29] | 2020 | 396 | - | CC | 0–59 months | Oromia | Simple random sampling technique |
| 51 | Girma M et al. [12] | 2018 | 469 | - | CC | 0–59 months | Amhara | Multi-stage sampling procedures |
| 52 | Brhanemeskel H [63] | 2021 | 155 | - | CC | 6–23 months | Addis Ababa | Simple random sampling technique |
| 53 | Getachew B et al. [17] | 2018 | 352 | - | CC | 0–59 months | Harari | Simple random sampling technique |
Descriptive summary of studies included in this systematic review and meta-analysis of the prevalence and risk factors of diarrhea among under-five children in Ethiopia (Ethiopia, 2017–2022).
CS: cross-sectional study; CCS: comparative cross-sectional study; CC: case-control study.
Meta-Analysis
The meta-analysis included only studies with a cross-sectional design. The overall pooled prevalence of diarrhea among under-five children in Ethiopia was found to be 20.8% (95% CI: 18.69–22.84, I2 = 94.9%, p < 0.001) (Figure 2).
FIGURE 2
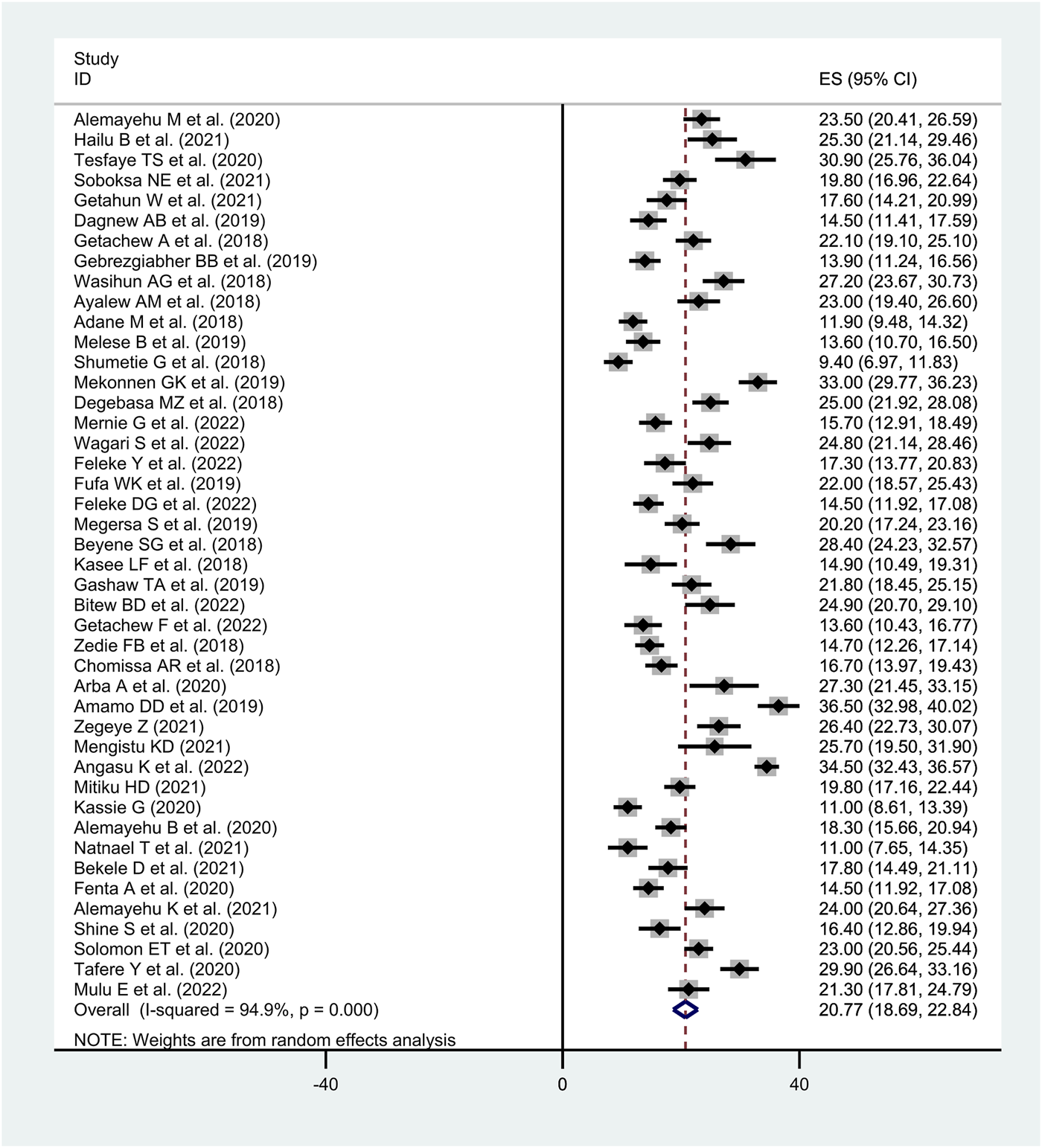
Forest plot of the pooled prevalence of diarrhea among under-five children in Ethiopia, (Ethiopia, 2017–2022).
We performed subgroup analysis based the studies’ geographical settings (i.e., region of the country). Accordingly, the highest proportion of acute diarrhea was observed in Gambella region with a prevalence of 33.0% (95% CI: 29.76–36.23) followed by SNNPR region with a prevalence of 24.2% (95% CI: 18.94–29.47), Dire Dawa city administration 23.0% (95% CI: 20.56–25.44), Oromia 22.6% (95% CI: 18.85–26.28), Amhara region 19.1% (95% CI: 15.95–22.14) and the lowest proportion was reported from Sidama region at 14.2% (95% CI: 12.37–16.11) (Table 2).
TABLE 2
| Sub-group | Number of studies (n) | Prevalence of diarrhea | 95%CI | I2 (%) | p-value |
| Sex | |||||
| Male | 16 | 19.73 | 15.39–24.07 | 94.1 | p < 0.001 |
| Female | 16 | 19.74 | 15.70–23.78 | 93.1 | p < 0.001 |
| Age of the child | |||||
| 0–5 months | 15 | 16.14 | 9.65–22.63 | 92.5 | p < 0.001 |
| 6–11 months | 19 | 25.34 | 21.36–29.32 | 83.0 | p < 0.001 |
| 12–23 months | 22 | 25.42 | 21.50–29.35 | 89.4 | p < 0.001 |
| 24–59 months | 20 | 19.56 | 15.37–23.75 | 94.9 | p < 0.001 |
| Regions | |||||
| Amhara | 16 | 19.05 | 15.95–22.14 | 93.4 | p < 0.001 |
| Oromia | 10 | 22.56 | 18.85–26.28 | 91.3 | p < 0.001 |
| SNNPR | 8 | 24.21 | 18.94–29.47 | 95.5 | p < 0.001 |
| Addis Ababa | 3 | 16.80 | 8.84–24.77 | 95.7 | p < 0.001 |
| Tigray | 2 | 20.49 | 7.46–33.53 | 97.1 | p < 0.001 |
| Sidama | 2 | 14.24 | 12.37–16.11 | 0.0 | 0.569 |
| Gambella | 1 | 33.00 | 29.76–36.23 | 0.0 | - |
| Beni Shangul Gumuz | 1 | 14.50 | 11.92–17.08 | 0.0 | - |
| Dire Dawa | 1 | 23.00 | 20.56–25.44 | 0.0 | - |
Subgroup analysis of acute diarrhea among under-five children in Ethiopia (Ethiopia, 2017–2022).
We conducted a stratification analysis on prevalence, considering age groups and gender. Our findings revealed that the prevalence of diarrhea among male under-five children in Ethiopia was 19.73% (95% CI: 15.39–24.07, I2 = 94.1%, p < 0.001), a rate comparable to that of female children. Additionally, our analysis revealed that the prevalence of childhood diarrhea varied across different age groups:0–5 months was 16.14% (95%CI: 9.65–22.63, I2 = 92.5%, p < 0.001), 6–11 months 25.34% (95%CI: 21.36–29.32, I2 = 83.0%, p < 0.001), 12–23 months 25.42% (95%CI: 21.50–29.35, I2 = 89.4%, p < 0.001), and 24–59 months 19.56% (95%CI: 15.37–23.75, I2 = 94.9%, p < 0.001) (Table 2 and Supplementary Material S4A–D).
Publication Bias
In this meta-analysis, possible publication bias was visualized through funnel plots and using Egger’s weighted regression test. Asymmetrical large, inverted funnel resembled the absence of publication biases (Figure 3). The Egger’s tests were also not statistically significant for the estimated prevalence of diarrhea in Ethiopia, with a p-value of 0.06.
FIGURE 3
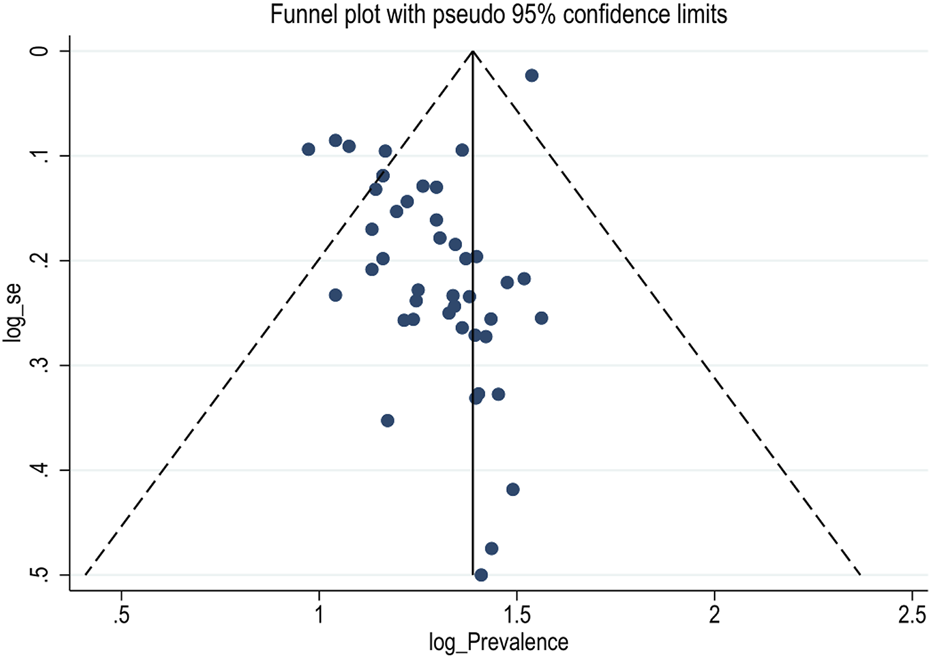
Funnel plot showing publication bias of prevalence of diarrhea among under-five children in Ethiopia, (Ethiopia, 2017–2022).
Sensitivity Analyses
To detect the influence of one study on the overall meta-analysis estimate, sensitivity analysis was conducted using a random-effects model. There was no evidence for the influence of a single study on the overall pooled result of diarrheal morbidity in Ethiopia (Supplementary Material S5).
Meta-Regression
We conducted a univariate meta-regression analysis by considering publication year, sample size, and quality score as covariates to identify the possible sources of heterogeneity across primary studies, but none of these variables was found to be a statistically significant source of heterogeneity. The results of meta-regression analysis also showed no significant relationship between prevalence of diarrheal disease and sampled size (p = 0.084) and publication year (p = 0.729).
Meta-Analysis of Selected Associated Factors and Evidence From the Reviewed Studies
Table 3 demonstrates the summary of the studies included in this review. The factors associated with acute diarrheal disease have been divided into categories of child, parental, and WASH and environmental factors.
TABLE 3
| Author | Year | Study design | Region | Risk factors and there strength of association | ||
|---|---|---|---|---|---|---|
| Child factors | Parental/household level factors | WASH and environmental factors | ||||
| Brhanu H et al. [11] | 2017 | Unmatched case-control | Benishangul Gumuz | • Child’s age (6–11 months) [AOR = 3.85, 95%CI (1.67, 8.86)] • Children from rural areas [AOR = 6.84, 95%CI = (3.26, 14.33)] • Bottle feeding [AOR = 10, 95% CI = (4.56, 21.94)] • Being wasted child [AOR = 2.25, 95%CI = (1.30, 3.91)] |
• No formal education [AOR = 4.13, 95% CI (1.54, 11.06)] • Mothers with a history of diarrhea [AOR = 8.81, 95%CI = (4.04, 19.20)] |
• Children did not use treated drinking water at home [AOR = 2.46, 95%CI (1.32, 4.57)] • Unsafe child stool disposal [AOR = 2.72, 95%CI = (1.54, 4.81)] |
| Derseh BT et al. [27] | 2021 | Unmatched Case-control | Amhara | • Hand washing without soap [AOR = 3.75, 95% CI: (1.16–12.13) • Average family monthly income [AOR = 6.22; 95% CI: 1.30, 29.64)] • Poor knowledge about acute diarrhea (AOR = 15.3; 95% CI: 4.18, 55.88) • Families who consume leftover food (AOR = 5.52; 95% CI: 1.60, 19.03) |
• Families who did not treat their drinking water at home (AOR = 9.36; 95% CI: 2.73, 32.08) • Families who dispose infant feces outside the latrine (AOR = 11.01; 95% CI: 3.37, 35.96) |
|
| Delelegn MW et al. [39] | 2020 | Unmatched Case-Control | Amhara | • Children who did not exclusively breastfeed [AOR = 3.32; 95% CI (1.206, 9.14)] • Children who consumed left-over food [AOR = 2.96; 95% CI (1.19, 7.32)] |
• Mothers/caregivers did not wash their hands at the critical time [AOR = 5.47; 95% CI:1.68, 17.8] • Mothers had not been counseled by health professionals [AOR = 3.23; 95% CI (1.15, 9.09)] • Mother who had a history of diarrhea in previous 2 weeks [AOR = 6.06; 95% CI (2.42, 15.22)] |
• Unsafe child stool disposal [AOR = 4.12; 95% CI (1.25, 13.5)] • Families did not treat drinking water [AOR = 2.85; 95% CI (1.27, 6.42)] |
| Mosisa D et al. [7] | 2021 | unmatched case-control | Oromia | • Child’s age (6–11 months) [(AOR 2.46; 95%CI: 1.09–5.57 • Child’s age (12–23 months) (AOR 3.3, 95% CI 1.68–6.46) • Not being vaccinated against rotavirus (AOR 2.45, 95% CI 1.25–4.81) |
• Mothers'/caregivers’ history of diarrheal diseases (AOR 7.38, 95% CI 3.12–17.44) • Mothers’/caregivers’ did not wash their hands during critical time (AOR 10.6, 95% CI 3.74–29.81) |
• Lack of a hand-washing facility near a latrine (AOR 5.22, 95% CI 3.94–26.49) • Improper domestic solid waste disposal (AOR 2.68, 95% CI 1.39–5.18) • Households who had not utilized latrines properly (AOR 2.34; 95%CI: 1.16, 4.75) |
| Baye A et al. [16] | 2021 | Matched case-control | Amhara | • Lack of exclusive breastfeeding (mOR = 2.12; 95% CI: 1.15–3.70) | • Age of mothers/caregivers (>35 years of age) (adjusted matched odds ratio [adjusted mOR] = 2.00; 95% CI: 1.37–5.8) • Marital status (divorced/widowed) [mOR = 1.40; 95% CI: 1.26–3.3] • Mothers/caregivers washed their hands at fewer than three critical times daily (mOR = 4.50; 95% CI: 2.54–9.50) |
• Presence of feces within/around latrines (mOR = 1.37; 95% CI: 1.21–3.50) • Lack of handwashing facility near latrine (mOR = 1.50; 95% CI: 1.30–5.30) • Presence of domestic sewage discharge within and/or outside the compound (mOR = 3.29; 95% CI: 1.85–7.50) |
| Soboksa NE et al. [29] | 2020 | Matched Case-Control | Oromia | • Number of under-five in the household (being single child) (AOR = 2.76; 95% CI: 1.33–5.71) | • Wealth status (middle (AOR = 5.39; 95% CI: 1.99–14.55) and rich AOR = 3.69; 95%CI: (1.36–10.01) • Mothers/caregivers who did not use soap for hand washing (AOR = 2.89; 95% CI: 1.35–6.15) • Children whose mothers/caregivers do not wash hands before water collection (AOR = 4.28; 95% CI: 1.46–12.56) |
• Family collected water from protected well/spring (AOR = 4.01; 95% CI: 1.40–11.44) • Families used the pit as a method of waste disposal (AOR = 4.91; 95% CI: 1.39–13.29) |
| Girma M et al. [12] | 2018 | Case-Control | Amhara | • Presence of two or more young siblings (AOR, 4.15; 95% CI: 2.57–6.70) • Rural residence (AOR,2.11 95% CI: 2.21–3.68) |
• Lack of hand washing at critical times (AOR, 2.38; 95% CI: 1.42–3.99) | • Unimproved water sources (AOR, 1.88; 95% CI: 1.17–3.03) • Deepening method to take water from a water storage container (AOR, 2.11; 95% CI: 1.28–3.47) • Unsafe child stool disposal (AOR, 1.90; 95% CI: 1.12–3.22) |
| Brhanemeskel H [63] | 2021 | Case-control | Addis Ababa | • Bottle feeding (AOR = 3.47, 95% CI: 1.08, 11.16) | ||
| Getachew B et al. [17] | 2018 | A community-based case-control study | Harari | • Lack of exclusive breastfeeding [AOR: 5.24, 95% CI: (2.46–11.15)] • Consumption of leftover food [AOR: 3.20, 95% CI: (1.26–6.51)] |
• Maternal/caregiver’s history of diarrhea [AOR: 4.26, 95% CI: (1.47–12.34)] |
• Lacks of home-based drinking water treatment [AOR: 4.27, 95% CI: (2.12–8.60)] • Presence of feces around latrine [AOR: 3.74, 95% CI: (1.88–7.44)] |
| Alemayehu M et al. [19] | 2020 | Community based CS | SNNPR | • Unvaccinated for “rotavirus” (AOR: 2.87, 95% CI: 1.86–4.44) |
• Mother’s or caretaker’s diarrhea history in the last 2 weeks (AOR: 6.74, 95% CI: 2.51–18.07) | • Non-availability of latrine (AOR: 2.77, 95% CI: 1.66–4.63) • Faeces seen around the pit hole or floor of latrine (AOR: 2.92, 95% CI: 1.38–6.19) • Improper kitchen waste disposal (AOR: 2.31, 95% CI: 1.26–4.24) • Unprotected drinking water source (AOR:1.81, 95% CI: 1.14–2.88) |
| Hailu B et al. [33] | 2021 | Community based CS | Amhara | • Playing with soil (AOR = 8.40; 95% CI: 4.58–36.66) • The habit of eating soil (AOR = 6.24; 95% CI: 1.99–19.78) |
• Poor handwashing habit after visiting toilet (AOR = 7.70; 95% CI: 2.71–21.79) • Not washing hands before feeding the child (AOR = 19.10; 95% CI: 5.46–66.52) |
• Absence of latrine (AOR = 3.87; 95% CI: 1.24–12.08) |
| Tesfaye TS et al. [34] | 2020 | Community-based CS | SNNPR | • The number of family members (AOR: 2.7, 95% CI [1.28–5.72]) • Poor hand washing practice during a critical time (AOR: 2.68, 95% CI [1.14–6.32]) |
• Presence of animals in households (AOR: 2.59, 95% CI [1.19–5.65]) • Absence of latrine (AOR: 2.13, 95% CI [1.01–4.50]) |
|
| Soboksa NE et al. [50] | 2021 | Comparative cross-sectional study | Oromia | • In CLTS kebeles: number of under-five children (≥2), (AOR: 3.36; 95%CI: 1.35–8.34) | • Families did not treat drinking water at home compared to those who treated in CLTS kebeles (AOR = 2.35; 95% CI: 1.02–5.98) • In non-CLTS kebeles: family size (≥5), (AOR: 2.93, 95%CI: 1.32–6.49); use of paper (AOR: 2.78, 95%CI: 1.41–8.34), and leaf (AOR:4.58, 95%CI: 2.01–16.47) as anal cleanse material |
|
| Getahun W et al. [41] | 2021 | Community-based CS | Amhara | • Poor handwashing practice at critical times (AOR = 1.85; 95% CI: 1.34–3.56) • No information about diarrhea being prevented by handwashing (AOR = 3.12; 95% CI: 1.64–6.27) |
• Water consumption of less than 20 L per capita per day ([AOR] = 2.45; 95% CI: 1.36–5.84) • Practicing unsafe child feces disposal (AOR = 2.51; 95% CI: 1.69–4.64) Unimproved sanitation facility (AOR = 3.57; 95 %CI: 1.64–6.51) |
|
| Dagnew AB et al. [38] | 2019 | A community-based CS | Amhara | • Not breastfeeding (AOR = 2.3 (1.023, 5.46) | • Poor hand washing practice (AOR = 6.104 (2.100, 17.738) | • Lack of hand washing facilities in the household (AOR = 3.910 (1.770, 8.634) • Lack of separate feeding materials (AOR = 5.769 (1.591, 9.220) |
| Getachew A et al. [51] | 2018 | A community-based CS | Amhara | • Children age (≤1 year) [AOR = 1.82, 95% CI(1.39, 4.63)] • Not breastfeeding [AOR = 3.13, 95% CI(1.62, 6.03)] |
• Unable to read and write [AOR: 4.37, 95%CI:1.72–11.08) | |
| Gebrezgiabher BB et al. [35] | 2019 | Comparative cross-sectional study | Tigray | • Mothers who had diarrhea in the 2 weeks preceding the survey (AOR = 3.3; 95% CI 1.97, 5.57) | • Non-model households (AOR = 1.9; 95% CI 1.01, 3.56) • Have proper waste disposal method (AOR = 2.6; 95% CI 1.25, 5.58) • Families who did not use latrine (AOR = 2.1; 95% CI 1.128, 3.897) |
|
| Wasihun AG et al. [42] | 2018 | A community based CS | Tigray | • Child age (36–47 months) [AOR = 2.57; 95% CI: 1.45–4.55] | • Mothers not hand washing at critical times [AOR = 15.42; 95% CI: 2.02–117.78] | • Improper solid waste disposal [AOR = 12.81; 95% CI: 2.50–65.62] • Unimproved source of drinking water [AOR = 3.69; 95% CI: 2.03–6.71] |
| Ayalew AM et al. [52] | 2018 | Comparative cross-sectional study | Amhara | • In ODF kebeles: water shortage (AOR: 8.75; 95% CI: 1.13–67.83) • In OD kebeles: water shortage (AOR: 18.47; 95% CI: 4.69–72.76) |
||
| Adane M et al. [30] | 2018 | Community based CS | Addis Ababa |
• Household size of six or more persons (AOR 2.3; 95% CI 1.4–3.9) • Low monthly household income (AOR 2.4; 95% CI 1.4–4.0) |
• | |
| Melese B et al. [24] | 2019 | Community based CS | Sidama | • Age (12–24 months) [AOR: 12, 95%CI: 1.78, 83.18] • Being under nourished [AOR: 6.41, 95% CI (2.47, 16.77.)] |
• Mother with no formal education [AOR: 3.97; 95%CI: 1.60, 8.81] • Not washing hands [AOR, 3.10, 95% CI (1.10, 8.67)] • Use of water only for hand washing [AOR: 6.41; 95%CI (2.51, 16.39)] • Hand washing after visiting latrine [AOR: 2.73, 95% CI (1.05, 6.56)] |
• Housing floor material (mud) [AOR: 3.22, 95% CI (1.16, 8.91] • Improper way of refuse disposal [AOR, 3.23, 95% CI (1.37, 7.60)] |
| Shumetie G et al. [14] | 2018 | Community based CS | Amhara | • No receipt of Rotavirus vaccine dose 2 [AOR = 3.96, 95%CI; 2.13, 7.33] • Non-exclusive breastfeeding [AOR = 2.69, 95%CI; 1.39, 5.19] |
• employed and private business occupational status of mothers [AOR = 2.10, 95%CI; 1.02, 4.31)] • Low household monthly income (less than Ethiopia Birr (ETB) 600) [AOR = 2.10, 95% CI; 1.2, 7.2] |
• Unavailability of solid waste disposal system [AOR = 2.62, 95%CI; 1.19, 5.77] |
| Mekonnen GK et al. [36] | 2019 | Comparative cross-sectional study | Gambella | • Child’s age (≤5 months), (AOR: 2.46, 95%CI: 1.26–4.81), 6–11 months (AOR: 2.09, 95%CI: 1.15, 3.79) | • Caregiver with no formal education (AOR: 2.59, 95%CI: 1.43, 4.71), primary school (AOR: 1.91, 95%CI: 1.03, 3.55) | • Use of surface water (AOR: 1.92, 95%CI: 1.24, 2.98) • Lack of latrine facility (AOR: 1.45, 95%CI: 1.07, 1.96) |
| Degebasa MZ et al. [53] | 2018 | Comparative cross-sectional study | Addis Ababa | • CLTSH implemented: number of undef-five children (≥2), (AOR:2.33, 95%CI: 1.09, 4.96) | • CLTSH implemented: mothers/caregivers had negative attitude toward diarrhea (AOR = 2.07; 95% CI: 1.06–4.04) | • CLTSH implemented: lack of clean water storage (AOR: 2.36, 95%CI: 1.16, 4.80) • Not CLTSH implemented kebeles: Feces in compound (AOR: 1.88, 95%CI: 1.1, 3.22), lack of hand washing facility (AOR: 2.64, 95%CI: 1.47, 4.74) |
| Mernie G et al. [40] | 2022 | Comparative cross-sectional study | Amhara | • In CLTSH-implementing areas, use of only water to wash hands (AOR: 3.28; 95% CI:1.13–9.58) and having a mother/caregiver who did not wash their hands at critical times (AOR: 3.02; 95% CI:1.12–8.12) were factors significantly associated with acute diarrhea • From the pooled analysis, not washing hands at critical times (AOR: 2.54; 95% CI:1.59–4.06) |
• In non-CLTSH-implementing areas, unimproved water source ([AOR]: 2.81; 95% CI:1.65–4.78), unsafe disposal of child feces (AOR: 2.10; 95% CI:1.13–3.89), improper solid waste disposal (AOR: 1.95; 95% CI:1.12–3.38), and untreated drinking water (AOR: 2.33; 95% CI:1.21–4.49) were factors significantly associated with acute diarrhea • From the pooled analysis, unsafe disposal of child feces (AOR: 2.20; 95% CI:1.34–3.60) and unimproved water source (AOR: 2.56; 95% CI:1.62–4.05) |
|
| Wagari S et al. [54] | 2022 | CS | Oromia | • Child’s age [6–11months, (adjusted prevalence ratio (APR) = 1.54; 95% CI: 1.03–2.29) and 12–23months, (APR = 1.58; 95% CI: 1.01–2.46) | ||
| Feleke Y et al. [15] | 2022 | CS | Oromia | • Child’s age [7–11months, AOR: 9:15, 95% CI (2.05, 40.71)], and 12–23 months [AOR: 4.98 (1.21, 20.56)] • No of under five children (two and above) [AOR: 2.20; 95%CI: (1.08, 4.49)] • Not exclusive breastfeeding history [AOR: 4.72, 95% CI (1.17, 19.13)] |
• Total family size (>5) [AOR: 5.042, 95% CI (2.326, 10.931)] • Washing hands with water only (AOR: 2.95; 95%CI 1.34, 6.48) |
|
| Fufa WK et al. [55] | 2019 | Comparative cross-sectional study | Oromia | • In urban households: Being male (AOR: 2.3; 95%CI:1.2, 4.7) | • In urban households: Poor knowledge on home management of diarrhea (AOR = 2.7(CI [1.3, 6.5]), difficulty in preparing oral rehydration salt (AOR = 4.0CI [1.4, 11.0) • In rural households: Poor knowledge on home management of diarrhea AOR = 13.4(CI [5.3, 34.0]), difficulty in preparing oral rehydration salt (AOR: 2.4; 95%CI:1.3, 5.3), easy to get Zinc (AOR: 2.4; 9%CI: 1.2, 5.0) |
|
| Feleke DG et al. [56] | 2022 | Community based CS | Amhara | • Initiation of supplementary food before 6 months (AOR = 6.49 (2.01–20.96)) | • Households with unclean latrine (AOR = 11.48 (5.64–23.35)) • Lack of hand washing facility (AOR: 7.07; 95%CI 3.84–13.03) Lack of solid waste disposal pit (AOR: 1.78, 95%CI: 0.95–3.36) |
|
| Megersa S et al. [57] | 2019 | Comparative cross-sectional study | Oromia | • In non ODF Sub-districts: age (12–23 months) (AOR: 3.71, 95%CI: 1.50, 9.19) | • In non ODF Sub-districts: Presence of faces in the compound (AOR: 2.10; 95%CI: 1.05, 4.17) | |
| Beyene SG et al. [58] | 2018 | Community based CS | Oromia | • Living in the home with single room [(AOR = 6.01, 95% CI(1.01, 36.01)] • Living with animal in the same house [AOR: 8.31, 95% CI(2.46, 28.06)] |
||
| Kasee LF et al. [59] | 2018 | Community-based CS | Oromia | • No regression analysis | ||
| Gashaw TA et al. [23] | 2019 | Community- based CS | SNNPR | • Being underweight (AOR: 3.14; 95%CI: 1.93, 5.11) • Not received full vaccination (AOR: 2.06, 95%CI: 1.021, 4.16) |
Mother education level (no education [(AOR: 3.89; 95%CI: 1.62–9.36)] | |
| Bitew BD et al. [31] | 2022 | Community-based CS | Amhara | • Child’s age (1–12months) [AOR: 9.22, 95%CI: (2.93–29.04)] and age group of 13–24 months [AOR: 4.44, 95%CI: (1.87–10.56)] | • Low monthly income (AOR: 3.68, 95% CI: (1.81–7.51)] Poor hand washing practice [AOR: 8.37, 95% CI: (3.12–22.52) |
|
| Getachew F et al. [60] | 2022 | Community-based CS | Addis Ababa | • | • Low household income (<1000 ETB)[AOR: 7.21, 95% CI (1.49, 34.92)] • Maternal history of diarrhea [AOR: 8.03, 95% CI (1.32, 48.67)] |
• Water storage duration (1 week and above) [AOR: 5.10, 95% CI (1.47, 17.62)] • Lack of hand washing facility [AOR: 5.70, 95% CI (1.01, 32.247)] Presence of uncollected garbage in the compound [AOR: 3.42, 95% CI (1.38, 8.49)] |
| Zedie FB et al. [28] | 2018 | Comparative cross-sectional study | Sidama | • Child’s age (12–23 months) [AOR: 3.74, 95%CI (1.23, 10.53)] | • Mothers with self or private employed [AOR: 5.08, 95%CI (1.88, 13.66)] • Mothers used soap sometimes when washing hands [AOR: 2.37, 95%CI (1.06, 5.27)], or did not use at all [AOR: 33.33, 95%CI (13.58, 76.98)] |
• Non- HDA (health development army) [AOR: 1.88, 95%CI (1.05, 3.37)] • Distance of latrine from the house [6 to 10, (AOR: 2.63, 95%CI: 1.16, 5.97), and above 10 m (AOR: 3.22, 95%CI (1.26, 8.24)] • Households that had no separate kitchen [AOR: 3.42, 95%CI (1.77, 6.62)] • Not treat water at home [AOR: 12.88 95%CI (1.42, 16.57)] |
| Chomissa AR et al. [20] | 2018 | A community based comparative cross-sectional | SNNPR | • Unvaccinated for Rota vaccine (AOR = 2.45; 95%CI: 1.48–4.04) | • Mothers who had history of diarrhea (AOR = 2.32; 95%CI: 1.28–4.17) | • Non-model households (AOR = 2.54; 95%CI: 1.55–4.17) • Households who did not have latrine (AOR: 3.07; 95%CI: 1.62–5.86) • Unsafe child stool disposal (AOR = 2.19; 95%CI: 1.32–3.64) |
| Arba A et al. [89] | 2020 | A community based cross-sectional | SNNPR | • - | • - | • - |
| Amamo DD et al. [62] | 2019 | A community based cross-sectional | Oromia | • Child’s age [6–11 months [AOR = 2.72; 95% CI(1.18, 6.27)], 12–23 months [AOR: 2.28; 95%CI (1.13, 4.62)], 24–35 months (AOR: 2.13; 95%CI: (1.21, 3.75)] • Number of under five children (two) [AOR = 1.527; 95% CI: (1.04, 2.24)] • Not exclusive breastfeed [AOR = 2.45; 95% CI: (1.61, 3.73)] • Early initiation of supplementary feeding (<6 months) [AOR = 2.16; 95% CI(1.22,.3.83)] • Not received pneumococcal vaccination [AOR = 6.72; 95% CI(1.20,.37.65)] • Not received Vitamin A supplementation [AOR = 1.66: 95% CI(1.04,.2.68)] |
• Maternal educational status (primary education) [AOR = 3.75, 95% CI: (1.07, 13.22)] | • Improper waste disposal method [AOR = 1.92; 95% CI: (1.26, 2.94)] |
| Zegeye Z [90] | 2021 | A community based cross-sectional | Amhara | • Family size (≥5 members (AOR = 2.48, 95%CI: 1.58, 3.89) • Use of water for hand washing (AOR = 3.45, 95%CI: 2.20, 5.41) |
Feacal mater seen around the pit-whole (AOR = 1.86, 95%CI: 1.19, 2.91) | |
| Mengistu KD [61] | 2021 | Health center based cross-sectional study |
Amhara | • Child feeding (children mostly taking adult food [AOR = 6.42 (1.09, 37.53)] • Duration of breastfeeding (<6 months) [AOR = 7.64 (1.51, 38.63) • Start supplementary feeding before less than 6 months [AOR = 9.764 (95% CI, 1.79, 53.071)] • Not taking measles vaccination [AOR = 4.78 (1.813, 12.647)] |
• Low monthly income (below 3874 ETB) [AOR = 19.45 (1.439, 263.059)] | |
| Angasu K et al. [6] | 2022 | Institution based cross-sectional study | SNNPR, Addis Ababa, and Oromia | • Child’s age (12–23 months) [AOR 4.37; 95%CI = 2.32–8.24] • Child feed by her/his own (AOR 2.4; 95%CI = 1.57–3.74) |
• Mothers or guardian with no formal education (AOR 3.55; 95%CI = 1.97–6.4) | • Washing child utensils by cool-water and soap (AOR 2.8; 95%CI = 2.12–3.57) Unprotected water (AOR 3.6; 95%CI = 2.13–6.13) |
| Mitiku HD [13] | 2021 | A community based cross-sectional study | Amhara | • Rural residency (AOR: 2.99; 95%CI: 1.30, 6.90) • Child not lived with biological mother (AOR:32.44, 95%CI: 14.07, 74.80) • Child live with currently pregnant of mothers (AOR: 5.66; 95%CI: 2.67, 11.99) • Not receiving measles vaccination (AOR: 3.91; 95%CI: 2.26, 6.77) |
• Not washing hands after visiting toilet (AOR = 7.91, 95% CI, 2.77, 22.59) | • Unprotected sources of drinking water (AOR: 14.01; 95%CI: 7.50, 26.15) • Sub-standard toilet facility (AOR: 2.74; 95%CI: 1.27, 5.89) |
| Kassie G [91] | 2020 | A community-based cross-sectional study | Amhara | |||
| Alemayehu B et al. [21] | 2020 | Community-based CS | Southwest Ethiopia | • Childs’s age (<6 months) (AOR 2.5; 95% CI 1.23–4.49) • Unvaccinated children for rotavirus prevention (AOR: 5.22, 95%Ci: 3.33–8.20) |
• Sharing of the residence with domestic animals (AOR: 2.87, 1.75–4.67) • Households obtaining water from unimproved sources (AOR: 2.53; 95%CI: 1.60–4.40) |
|
| Natnael T et al. [9] | 2021 | Community based CS | Amhara | • Child’s age (12–23 months) (AOR: 4.68, 95% CI: 1.45–1.50) • Presence of two or more under-five children in the house (AOR = 2.84, 95% CI: 1.19–6.81) |
• Unimproved water sources (AOR = 2.97, 95% CI: 1.28–6.87) • Presence of feces around the pit hole/slab/floor of the latrine (AOR = 3.34, 95% CI: 1.34–8.31) |
|
| Bekele D et al. [22] | 2021 | A community-based comparative cross-sectional study | Oromia | • Household health extension program implementation: Not vaccinated against Rotavirus (AOR: 49.8; 95% CI: 4.2–94.8); child not supplemented with vitamin A (AOR: 3.2; 95%CI:1.4–7.2) • Non-model families: child not vaccinated against Rotavirus (AOR: 10.9; 95%CI: 2.9–41.1) |
• Non-model families: Family size >5 (AOR [95% CI] = 5.2 [1.7–17.6]) | • Household health extension program implementation: model household (AOR: 2.4; 95%CI: 1.15, 5.00) • Unimproved water sources (AOR: 5.5; 95%CI: 2.2,-97.7) Non-model families: Unimproved water sources (AOR [95% CI] = 7.2 [1.6–13.2]), not using latrine (AOR [95% CI] = 6 [1.8–20.6]) |
| Fenta A et al. [43] | 2020 | A community-based CS | Beni Shangul Gumuz | • Complementary feeding before 6 months (AOR = 6.49, 95%CI: 2.01–20.96) | • Poor handwashing practice at a critical time (AOR = 5.92, 95%CI: 2.58–13.70) | • Poor latrine hygiene (AOR = 11.48, 95%CI: 5.64–23.35) • No handwashing facilities near latrines (AOR = 7.07, 95%CI:3.84–13.03) • Water storage (AOR = 8.6, 95%CI: 1.51–48.84) |
| Alemayehu K et al. [8] | 2021 | A community based CS | Oromia | • Child’s age: 6–11 months, (AOR: 1.55; 95%CI: 1.68, 3.52), and 12–23 months (AOR: 1.48; 95%CI: 1.84, 2.63) • Children who were not vaccinated against measles (AOR: 4.73, 95% CI: 2.43, 9.20) • Having two or more siblings (AOR: 3.11, 95% CI (1.81, 5.35) |
• Poor knowledge of mothers/caretakers on diarrhea prevention methods (AOR: 2.05, 95% CI (1.14, 3.69) Poor wealth index (AOR: 2.41, 95% CI (1.29, 4.51) |
• Inappropriate liquid waste disposal (AOR: 3.73; 95%CI: 1.94, 7.42) • Unsafe child feces disposal (AOR: 3.75; 95% CI (1.91, 7.39) |
| Shine S et al. [18] | 2020 | A community-based CS | Amhara | • Child’s age (7–11 months) (AOR: 4.2, 95% CI: 1.2–15.3) • Being the second-born child (AOR: 3.9, 95%CI: 1.8–8.5) • Not vaccinated against rotavirus (AOR: 10.3, 95%CI: 3.2–91.3) |
• Feeding children by hand (AOR: 2.5, 95%CI: 1.1–6.1) | |
| Solomon ET et al. [44] | 2020 | A community-based CS | Dire Dawa | • Maternal diarrhea (AOR = 2.22, 95% CI 1.10–4.47) • Not handwashing after contact with child feces (AOR = 6.27, 95% CI 2.01–19.55) |
• Use of a dipper to draw water from containers (AOR = 2.88, 95% CI 1.41–5.89) • Lack of refuse disposal facility (AOR = 2.47, 95% CI 1.09–5.60) |
|
| Tafere Y et al. [26] | 2020 | A community-based comparative cross-sectional study | Amhara | • Age of the child (>12 months) (AOR: 2, 95% CI (1.4, 2.7) | • Not attending formal education (AOR: 2.1, 95% CI (1.2, 2.7) • Family size ≥5 (AOR: 1.3, 95% CI (1.11, 1.9) • Low monthly income (AOR: 2.1, 95% CI (1.3, 2.) • Mothers had a diarrheal diseases in the last 2 weeks (AOR: 1.2; 95%CI: 1.1, 24) • Not handwashing after touching infants’ faeces (AOR:1.6; 95%CI: 1.12, 2.03) |
• Status of kebeles NODF [AOR: 2.4; 95%CI: (1.17, 3.23)] • Number of rooms in the house (1–2 rooms) [AOR: 1.3; 95%CI (1.1, 1.7)] • Type of house roof constructed (grass) [AOR: 2.3; 95%CI: (1.53, 3.4)] • Latrine utilization pattern [Sometimes, AOR: 1.50; 95%CI(1.1, 2.4)] • Lack of functional handwashing facility near the latrine (AOR: 11; 95%CI:8.1, 29.6) • Unsafe child stool disposal (AOR: 1.4; 95%CI: 1.1, 3.7) • Old latrine (>4 years) (AOR: 1.8; 95%CI: 1.2, 3.1) • Faeces seen around the compound 27.6 (18.9, 37)- |
| Mulu E et al. [32] | 2022 | Community-based cross-sectional study | SNNPR | • Bottle feeding [AOR: 8.27, 95% (1.09, 62.97)] | • Mothers/care takers who feed adult food to the children [AOR: 6.98, 95% (1.07, 45, 43)] | • Unimproved toilet facility feces seen outside the pit hole of latrines [AOR: 2.94, 95% (1.35, 6.43)] • Unimproved water source [AOR: 4.47, 95% CI (1.96, 10.21)] • Distance to water source (>1 h) [AOR: 2.25, 95% (1.14, 4.45)] |
Summary of risk factors and there strength of association of acute diarrhea among under-five children in Ethiopia, (Ethiopia, 2017–2022).
Child Related Factors
The most consistent factors associated with diarrhea, as shown in Table 3 were the child’s age, feeding status, nutritional status, and vaccination status for rotavirus.
Several studies consistently reported the highest prevalence of diarrhea among children occurred between one and 12 months of age [7, 8, 11, 15, 18, 31, 36, 54, 62]. Studies also found that bottled-fed children [11, 32, 63], not breastfeeding [38, 51], lack of exclusive breastfeeding [14–17], and starting complementary feeding before 6 months [61, 62] were the commonly identified associated factors with childhood diarrheal disease. Moreover, not being vaccinated against rotavirus [7, 14, 18–22], playing with soil and a habit of eating soil [33], being underweight [23, 24], and not receiving Vitamin A supplementation [22, 62] were factors associated with higher risk of diarrheal disease. Three studies found an increased risk of diarrheal disease among children living in households with two and above under-fives [50, 53, 62] (Table 3).
Supplementary Material S4A–F presents the pooled odds ratios of the association between child age, the child’s sex, and childhood diarrhea in Ethiopia. As depicted in Supplementary Material S6A, there is no statistically significant difference in the odds of experiencing childhood diarrheal disease between male and female children under the age of five (pooled OR = 0.61; 95% CI: 0.35–1.07, I2 = 88.0%, p < 0.001).
The likelihood of childhood diarrhea was 1.79 times higher among children aged 0–5 months (pooled OR = 1.79, 95% CI: 1.05–3.05, I2 = 60.8%, p = 0.006), 2.40 times higher among children aged 6–11 months (pooled OR = 2.40, 95% CI: 1.77–3.25, I2 = 38.3%, p = 0.071), and 1.65 times higher among children aged 12–23 months (pooled OR = 1.65, 95% CI: 1.07–2.54, I2 = 73.1%, p < 0.001) compared with children aged 48–59 months (Supplementary Material S6B–D).
However, there is no statistically significant association observed between diarrhea occurrence among children aged 24–35 months (pooled OR = 1.16, 95% CI: 0.89–1.51, I2 = 27.5%, p = 0.168) and 36–47 months (pooled OR = 1.15, 95% CI: 0.73–1.80, I2 = 62.1%, p = 0.015) when compared with those aged 48–59 months (Supplementary Material S6E, F).
Parental and Household Factors
Across the studies, the lack of maternal education [6, 11, 23, 24, 26, 62], poor maternal knowledge about diarrheal disease and its prevention measures [8, 27], and mothers poor handwashing practices (i.e., not washing their hands at the critical times, such as after visiting toilet, after touching children’s feces) [7, 12, 13, 26, 31, 33, 34, 38–44] were identified associated factors with acute diarrheal disease (Table 3).
This meta-analysis’s findings showed that there was association between mothers not washing their hands after using the toilet with children having diarrhea (pooled OR = 3.05; 95% CI:2.08–4.54, I2 = 75.3%, p < 0.001, n = 15) (Supplementary Material S7A). Four studies [6, 15, 30, 54] used different reference categories, and we pooled the confounder adjusted odds ratios separately. Similarly, the odds of childhood diarrhea was lower by 51% among children whose mothers washed their hands at the critical time (pooled OR = 0.49, 95% CI: 0.31–0.70, I2 = 48.6%, p = 0.120, n = 4).
Mothers’ history of diarrheal episodes in the previous 2 weeks before the study was also identified as a risk factor for childhood diarrheal disease [7, 11, 17, 19, 20, 26, 27, 35, 39, 60, 64]. The overall result of the meta-analysis revealed that children whose mothers had a history of diarrhea in the previous 2 weeks were three times more likely to develop diarrhea than children without a history of maternal diarrhea in the previous 2 weeks (pooled OR = 3.19, 95%CI: 1.94–5.25, n = 13). We used a random effect meta-analysis model to estimate pooled OR because the included studies had high heterogeneity (I2 = 79.4%, p-value = 0.006) (Supplementary Material S7B). Since two studies [28, 54] used different reference categories, we pooled the odds ratios separately. Likewise, the odds of diarrhea was reduced in children whose mothers had not recently experienced any diarrheal episode (pooled OR = 0.49, 95% CI: 0.35–0.68, I2 = 0.0%, p = 0.901, n = 2).
Water, Sanitation and Hygiene (WASH) Factors
As shown in Table 3, the most consistent WASH related factors associated with diarrheal disease in Ethiopia were i) the lack of toilet facility or use of unimproved toilet facility [7, 19, 20, 32–36], ii) the poor latrine hygienic condition [9, 16, 17, 19, 26, 43, 56], iii) unsafe child stool disposal [11, 12,19, 20, 26, 27, 39–41], iv) poor domestic solid waste disposal [7, 8,14, 19, 40, 42, 56, 62], v) not treating drinking water [11, 27, 28, 39, 50], vi) collecting water from unimproved drinking sources [6, 9, 12, 13, 19, 21, 32, 40, 42], and vii) lacked handwashing facilities near the toilet facilities [12, 38, 56, 60].
The pooled result of this meta-analysis indicated that children from households that lacked handwashing facilities near the toilet were 4.16 times more likely to have diarrheal morbidity as compared to their counterparts (pooled OR = 4.16, 95%CI: 2.49–6.95, I2 = 71.1%, p = 0.001, n = 9) (Supplementary Material S7C). Children living in households without latrine facilities were found to be 1.56 times more likely to develop diarrhea than children living in households with latrine facilities (pooled OR = 1.56, 95%CI: 1.05–2.33, I2 = 71.1%, p = 0.001, n = 9) (Supplementary Material S7D).
The meta-analysis results of ten studies that reported confounder adjusted association between household water treatment and childhood diarrhea. The overall result showed that, children from households that did not use treated drinking water at home were two times higher odds of having diarrheal disease compared to children living in households that used home treated drinking water (pooled OR = 2.28, 95% CI: 1.50–3.46, I2 = 64.5%, p = 0.003, n = 10) (Supplementary Material S7E).
Discussion
Childhood diarrheal disease can cause significant morbidity and remain a significant public health issue across the world. This systematic review and meta-analysis aimed to estimate the pooled prevalence of diarrhea disease and summarize the potential associated factors among under-fives in Ethiopia. The pooled prevalence showed that one in every five children under the age of 5 years in Ethiopia experienced diarrheal disease. Overall, early childhood, poor child nutrition status, use of unimproved sanitation, lack of hand washing facilities, maternal diarrhea illness in previous 2 week, inadequate maternal hygiene knowledge and behavior, and improper domestic solid waste disposal were important factors associated with diarrheal disease.
The meta-analysis findings from the included 44 studies indicated that 20.8% of children under five in Ethiopia experienced diarrheal diseases. The results are comparable with the 2016 Ugandan Demographic and Health Survey (DHS) that reported the prevalence of diarrhea among children <5 years in Uganda to be 20% [65]. Our finding was in line with the prior pooled prevalence estimates of diarrhea 22% [37]. Although the results showed a relatively lower prevalence, this figure seemed to imply that the prevalence of diarrheal disease has not dropped significantly. As such, it would be reasonable to argue that diarrheal disease was still a significant public health concern of high magnitude in Ethiopia. On the other hand, our finding showed that the diarrheal disease prevalence was higher than the overall prevalence of diarrheal disease among under-fives in sub-Saharan Africa 15.3% [66] and the East Africa pooled estimate 14.28% [67]. The observed higher prevalence of diarrheal disease in this review compared to the East Africa regional estimate could be due to the differences in methodological approaches. The DHS sampling, design, and setting were very different from the type of analysis that was used in the current study. The results of DHS reports are based on primary data collection, whereas we reviewed and used prevalence measurements from previously conducted primary studies from different areas, populations, and seasons.
The top three regions with the highest prevalence of diarrhea were observed in the Gambella region, 33.0%, SNNPR region 24.21%, and Dire Dawa city administration 23.0%, according to the subgroup analysis of this study. The Sidama region had the lowest proportion of diarrheal disease, at 14.24%. Regional estimates could differ due to differences in basic sanitation, household behavioral characteristics, and access to healthcare facilities. The high prevalence of diarrheal disease in the Gambella region could also be due to the inclusion of a single comparative cross-sectional study in sub-group meta-analysis, which was limited to a single area and did not represent the entire region. Corresponding to the Ethiopia DHS 2016 report, the Gambela region had the highest diarrheal morbidity in Ethiopia (14.5%), followed by the SNNP region (13.9%) [4].
The findings of our review suggest that there is no significant difference in the prevalence of childhood diarrheal disease between male and female children under the age of five. This observation aligns with previous studies that have also failed to identify gender as a significant risk factor for diarrheal illness [68]. Our study adds to the body of evidence indicating that diarrheal disease does not exhibit a gender-specific pattern among young Ethiopian children. Our review also highlights a notably high prevalence of diarrheal disease among children aged 12–23 months. Several factors may contribute to the heightened susceptibility in this age group, including increased mobility and exploratory behavior, which lead to greater exposure to contaminated environments [69].
The current study found lack of exclusive breastfeeding to be associated with a higher likelihood of diarrheal disease in children [14–17]. Evidence showed that breastfeeding is an ideal food for infants and young children and it prevents nearly half of all diarrheal episodes and 72% of diarrheal-related hospitalizations [70, 71]. Breast milk is safe, clean, and contains antibodies which help to protect children against many common childhood illnesses [72]. A meta-analyses of eighteen studies showed that not breastfeeding resulted in an excess risk of diarrheal mortality in comparison to exclusive breastfeeding among infants 0–5 months of age and to any breastfeeding among children aged 6–23 months [70]. This study identified significant association between being underweight [26, 27] and wasting [14] and childhood diarrhea. The relationship between child undernutrition and diarrheal illnesses was clearly shown [73] as diarrheal disease and malnutrition being bidirectionally related, meaning that: diarrhea causes malnutrition, and malnutrition worsens the course of diarrheal disease [74].
Across the studies, lack of maternal education was consistently associated with higher odds of childhood diarrheal [6, 11, 23, 24, 26, 62], which is consistent with research conducted in Nigeria [75]. The educational attainment of mothers influences hygienic practices, child feeding, weaning, and sanitation practices, all of which are crucial factors against the onset of childhood diarrheal disease. Primary studies identified mothers’ poor handwashing practices [7, 12, 13, 26, 31, 33, 34, 38–44] and mothers’ history of diarrheal episodes in the previous 2 weeks [7, 11, 17, 19, 20, 26, 27, 35, 39, 60, 64] as potential factor associated with childhood diarrhea. The result of this meta-analysis revealed that, the odds of childhood diarrhea was three times higher among children whose mothers did not practice hand washing at critical times. Likewise, the odds of childhood diarrhea among children whose mother had a history of diarrhea in the previous 2 weeks was three times higher than their counterparts. The importance of proper handwashing at critical times with appropriate handwashing agents has long been established in the reduction of diarrheal disease with handwashing with soap reducing the burden significantly [76–78]. For instance, a recent systematic review, handwashing promotion in communities prevents one-quarter of diarrhea episodes in Low and Middle-Income Countries (LMICs) [79]. The pooled result of this meta-analysis indicated that children from households that lacked handwashing facilities near the toilet were four times more likely to have diarrheal morbidity as compared to their counterparts. This finding supports previous studies which have identified lack of a handwashing station as predictors of diarrheal disease [12, 38, 56, 60]. This finding was not surprising as several studies explore strong link between handwashing and childhood diarrhea [80]. A systematic review of the literature by Shah et al. also reported handwashing are effective strategy for preventing all causes of diarrheal disease [81].
Children living in households that used untreated drinking water and those collecting water from unimproved drinking water sources were at a higher risk of diarrheal disease. Several studies provided evidence that improving access to safe drinking water reduces the risk of diarrheal disease in children [25, 82]. In our meta-analysis, the odds of developing childhood diarrhea in households that did not use treated drinking water at home were two fold higher compared to children used home treated drinking water, and that improved water quality was generally is an effective strategy in preventing diarrheal disease [83]. Although evidence exists that improved sanitation is one of the key factors in the reduction of diarrheal diseases [52, 84], however, to establish the effect of improved latrines on diarrheal disease prevention in the absence of universal or at least adequate latrine coverage in a given community is controversial and difficult as indicated in the recent cluster-randomized controlled trial on sanitation interventions reporting that improved latrines had no protective effect against childhood diarrheal prevalence [85, 86]. However, other systematic review and meta-analysis have shown that both improved neighborhood sanitation conditions and household sanitation are associated with reduced diarrheal illness [87].
Limitations
First, in the majority of the included studies diarrheal disease was based on self-reported screening and was not further clinically confirmed. Second, our study could lack representativeness at a country level as we did not find a study from some regions of Ethiopia. Third, there was high heterogeneity between included studies, as indicated by the I2 statistic. Fourth, we were unable to pool all the adjusted odds ratio as a number of the included studies showed significant heterogeneity and used different reference category. As a result, this work only summarizes the findings as described in the study protocol. Fifth, because the studies in this review were all observational in design, it is possible that other confounding variables could influence the identified associated factors. Sixth, the majority of included articles were observational studies (i.e., cross-sectional studies), so causality cannot be inferred. Last but not least, because our search was restricted to publicly accessible databases, we were unable to include databases like EMBASE.
Conclusion
Our study indicated that approximately one in five under-five children in Ethiopia experienced diarrheal disease during the 2 weeks, and there were regional variations in diarrheal prevalence among under-five children. The key contributing factors to childhood diarrheal were related to child, parental, and WASH factors (such as poor handwashing practices, use of untreated drinking, household that lacked handwashing facility near the toilet facility, and lack of toilet facility). To address diarrheal disease and achieve the Sustainable Development Goals and the 2030 agenda [88], Ethiopia must strive to strengthen current strategies and consider the identified factors. To reduce diarrheal disease among children under the age of five, priority should be given to strengthening interventions that focus on improving household WASH facilities and raising awareness about the importance of handwashing, proper sanitation, and hygiene practices.
Statements
Author contributions
BS: Conceptualization, Formal analysis, Investigation, Methodology, Writing–original draft. DA: Investigation, Methodology; Writing–review and editing. LM, PP, AK, YT, FD, DZ, TM, and DG: Visualization, Validation, Writing–review and editing. KA: Supervision, Visualization, Validation, Writing–review and editing. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare(s) that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank Madda Walabu University Goba Referral Hospital Public Health Department staff for providing their unreserved support.
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2024.1606399/full#supplementary-material
References
1.
Vos T Lim SS Abbafati C Abbas KM Abbasi M Abbasifard M et al Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet (2020) 396(10258):1204–22. 10.1016/S0140-6736(20)30925-9
2.
Troeger C Forouzanfar M Rao PC Khalil I Brown A Reiner RC et al Estimates of Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoeal Diseases: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis (2017) 17(9):909–48. 10.1016/S1473-3099(17)30276-1
3.
Ugboko HU Nwinyi OC Oranusi SU Oyewale JO . Childhood Diarrhoeal Diseases in Developing Countries. Heliyon (2020) 6(4):e03690. 10.1016/j.heliyon.2020.e03690
4.
EDHS. Central Statistical Agency (CSA) [Ethiopia] and ICF. 2016. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF (2016).
5.
EDHS. Central Statistical Authority [Ethiopia] and ORC Macro. 2001. Ethiopia Demographic and Health Survey 2000. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Authority and ORC Macro (2000).
6.
Angasu K Dame KT Negash A . Diarrheal Morbidity and Associated Factors Among Under-Five Children in Southwest Ethiopia: Institution Based Cross-Sectional Study. Durham, NC, United States: Research Square (2022). Available from: https://www.researchsquare.com/article/rs-1210060/v1 (Accessed June 15, 2022).
7.
Mosisa D Aboma M Girma T Shibru A . Determinants of Diarrheal Diseases Among Under Five Children in Jimma Geneti District, Oromia Region, Ethiopia, 2020: A Case-Control Study. BMC Pediatr (2021) 21(1):532. 10.1186/s12887-021-03022-2
8.
Alemayehu K Oljira L Demena M Birhanu A Workineh D . Prevalence and Determinants of Diarrheal Diseases Among Under-Five Children in Horo Guduru Wollega Zone, Oromia Region, Western Ethiopia: A Community-Based Cross-Sectional Study. Can J Infect Dis Med Microbiol (2021) 2021:5547742. 10.1155/2021/5547742
9.
Natnael T Lingerew M Adane M . Prevalence of Acute Diarrhea and Associated Factors Among Children Under Five in Semi-Urban Areas of Northeastern Ethiopia. BMC Pediatr (2021) 21(1):290. 10.1186/s12887-021-02762-5
10.
Sahiledengle B Kumie A Atlaw D Tekalegn Y Woldeyohannes D Zenbaba D et al The Role of Household Flooring on Childhood Diarrhea Among Children 0 to 23 Months of Age in Ethiopia: A Nationally Representative Cross-Sectional Study Using a Multi-Level Mixed Effect Analysis. Environ Health Insights (2021) 15:11786302211064423. 10.1177/11786302211064423
11.
Brhanu H Negese D Gebrehiwot M . Determinants of Acute Diarrheal Disease Among Under-Five Children in Pawi Hospital, Northwest Ethiopia. Am J Pediatr (2017) 3(6):68. 10.11648/j.ajp.20170306.12
12.
Girma M Gobena T Medhin G Gasana J Roba KT . Determinants of Childhood Diarrhea in West Gojjam, Northwest Ethiopia: A Case Control Study. Pan Afr Med J (2018) 30:234. 10.11604/pamj.2018.30.234.14109
13.
Mitiku HD . Determinates of Diarrhea Among Under-Five Children in Northwest Ethiopia. IJIER (2021) 9(5):92–103. 10.31686/ijier.vol9.iss5.3074
14.
Shumetie G Gedefaw M Kebede A Derso T . Exclusive Breastfeeding and Rotavirus Vaccination Are Associated With Decreased Diarrheal Morbidity Among Under-Five Children in Bahir Dar, Northwest Ethiopia. Public Health Rev (2018) 39:28. 10.1186/s40985-018-0107-6
15.
Feleke Y Legesse A Abebe M . Prevalence of Diarrhea, Feeding Practice, and Associated Factors Among Children Under Five Years in Bereh District, Oromia, Ethiopia. Infect Dis Obstet Gynecol (2022) 2022:4139648. 10.1155/2022/4139648
16.
Baye A Adane M Sisay T Hailemeskel HS . Priorities for Intervention to Prevent Diarrhea Among Children Aged 0–23 Months in Northeastern Ethiopia: A Matched Case-Control Study. BMC Pediatr (2021) 21(1):155. 10.1186/s12887-021-02592-5
17.
Getachew B Mengistie B Mesfin F Argaw R . Factors Associated With Acute Diarrhea Among Children Aged 0-59 Months in Harar Town, Eastern Ethiopia. East Afr J Health Biomed Sci (2018) 2(1):26–35.
18.
Shine S Muhamud S Adanew S Demelash A Abate M . Prevalence and Associated Factors of Diarrhea Among Under-Five Children in Debre Berhan Town, Ethiopia 2018: A Cross Sectional Study. BMC Infect Dis (2020) 20(1):174. 10.1186/s12879-020-4905-3
19.
Alemayehu M Alemu T Astatkie A . Prevalence and Determinants of Diarrhea Among Under-Five Children in Benna Tsemay District, South Omo Zone, Southern Ethiopia: A Community-Based Cross-Sectional Study in Pastoralist and Agropastoralist Context. Adv Public Health (2020) 2020:1–11. 10.1155/2020/4237368
20.
Abdi Reshid C. Prevalence and Associated Risk Factors of Diarrhea in Under-Five Children Among Health Extension Model and Non-Model Kebeles in Getta District, Southern Ethiopia: A Community Based Comparative Cross-Sectional Study. Master’s thesis. Jimma, Ethiopia: Jimma University (2018).
21.
Alemayehu B Ayele BT Kloos H Ambelu A . Individual and Community-Level Risk Factors in Under-Five Children Diarrhea Among Agro-Ecological Zones in Southwestern Ethiopia. Int J Hyg Environ Health (2020) 224:113447. 10.1016/j.ijheh.2019.113447
22.
Bekele D Merdassa E Desalegn M Mosisa G Turi E . Determinants of Diarrhea in Under-Five Children Among Health Extension Model and Non-Model Families in Wama Hagelo District, West Ethiopia: Community-Based Comparative Cross-Sectional Study. J Multidiscip Healthc (2021) 14:2803–15. 10.2147/JMDH.S324846
23.
Gashaw TA . Prevalence and Determinate Factors of Diarrhea Morbidity Among Under Five Children in Shake Zone, Southwest Ethiopia, a Community Based Cross-Sectional Study. Arch Community Med Public Health (2019) 5(1):008–14. 10.17352/2455-5479.000046
24.
Melese B Paulos W Astawesegn FH Gelgelu TB . Prevalence of Diarrheal Diseases and Associated Factors Among Under-Five Children in Dale District, Sidama Zone, Southern Ethiopia: A Cross-Sectional Study. BMC Public Health (2019) 19(1):1235. 10.1186/s12889-019-7579-2
25.
Kumie A . The Effect of Improved Water and Sanitation on Diarrhea: Evidence From Pooled Ethiopia Demographic and Health Surveys – A Multilevel Mixed-Effects Analysis. Ethiop J Health Dev (2020) 34(4).
26.
Tafere Y Abebe Abate B Demelash Enyew H Belete Mekonnen A . Diarrheal Diseases in Under-Five Children and Associated Factors Among Farta District Rural Community, Amhara Regional State, North Central Ethiopia: A Comparative Cross-Sectional Study. J Environ Public Health (2020) 2020:6027079. 10.1155/2020/6027079
27.
Derseh BT Tafese NM Panari H Bilchut AH Dadi AF . Behavioral and Environmental Determinants of Acute Diarrhea Among Under-Five Children From Public Health Facilities of Siyadebirena Wayu District, North Shoa Zone, Amhara Regional State, Ethiopia: Unmatched Case-Control Study. PLOS ONE (2021) 16(11):e0259828. 10.1371/journal.pone.0259828
28.
Berhe Zedie F Hailu Kassa D . Socio-Economic, Behavioral and Environmental Factors Associated With Diarrhea Among Under Five Children in Health Development and Non-Health Development Army Member Mothers in Wondogenet, South Ethiopia. Health Educ Care (2018) 3(3):1–8. 10.15761/hec.1000144
29.
Soboksa NE Gari SR Hailu AB Alemu BM . Association Between Microbial Water Quality, Sanitation and Hygiene Practices and Childhood Diarrhea in Kersa and Omo Nada Districts of Jimma Zone, Ethiopia. PLOS ONE (2020) 15(2):e0229303. 10.1371/journal.pone.0229303
30.
Adane M Mengistie B Mulat W Medhin G Kloos H . The Most Important Recommended Times of Hand Washing With Soap and Water in Preventing the Occurrence of Acute Diarrhea Among Children Under Five Years of Age in Slums of Addis Ababa, Ethiopia. J Community Health (2018) 43(2):400–5. 10.1007/s10900-017-0437-1
31.
Bitew BD Getachew A Azanaw J . Diarrhea Prevalence and Associated Factors Among Under-Five Children in the Periphery Area of Azezo Sub-City, Gondar, Northwest Ethiopia: A Community Based Cross-Sectional Study. Durham, NC, United States: Research Square (2021). Available from: https://www.researchsquare.com/article/rs-1129227/v1 (Accessed June 15, 2022).
32.
Mulu E Nigusie A Endehabtu BF . Prevalence and Factors Associated With Acute Diarrheal Disease Among Under-Five Children in Southern Ethiopia: Community Based Cross Sectional Study. Durham, NC, United States: Research Square (2020). Available from: https://www.researchsquare.com/article/rs-28183/v1 (Accessed June 15, 2022).
33.
Hailu B Ji-Guo W Hailu T . Water, Sanitation, and Hygiene Risk Factors on the Prevalence of Diarrhea Among Under-Five Children in the Rural Community of Dangila District, Northwest Ethiopia. J Trop Med (2021) 2021:2688500. 10.1155/2021/2688500
34.
Tesfaye TS Magarsa AU Zeleke TM . Moderate to Severe Diarrhea and Associated Factors Among Under-Five Children in Wonago District, South Ethiopia: A Cross-Sectional Study. Pediatr Health Med Ther (2020) 11:437–43. 10.2147/PHMT.S266828
35.
Gebrezgiabher BB Abraha TH Tetemke D Gidey G Asres N Tesfamariam A et al Diarrheal Disease in Under-Five Children Among Model and Non-Model Families in Northern Ethiopia, 2017: A Comparative Cross-Sectional Study. BMC Res Notes (2019) 12(1):300. 10.1186/s13104-019-4322-0
36.
Mekonnen GK Alemu BM Mulat W Sahilu G Kloos H . Risk Factors for Acute Childhood Diarrhea: A Cross-Sectional Study Comparing Refugee Camps and Host Communities in Gambella Region, Ethiopia. Trav Med Infect Dis (2019) 31:101385. 10.1016/j.tmaid.2019.02.003
37.
Alebel A Tesema C Temesgen B Gebrie A Petrucka P Kibret GD . Prevalence and Determinants of Diarrhea Among Under-Five Children in Ethiopia: A Systematic Review and Meta-Analysis. PLOS ONE (2018) 13(6):e0199684. 10.1371/journal.pone.0199684
38.
Dagnew AB Tewabe T Miskir Y Eshetu T Kefelegn W Zerihun K et al Prevalence of Diarrhea and Associated Factors Among Under-Five Children in Bahir Dar City, Northwest Ethiopia, 2016: A Cross-Sectional Study. BMC Infect Dis (2019) 19(1):417. 10.1186/s12879-019-4030-3
39.
Delelegn MW Endalamaw A Belay GM . Determinants of Acute Diarrhea Among Children Under-Five in Northeast Ethiopia: Unmatched Case–Control Study. Pediatr Health Med Ther (2020) 11:323–33. 10.2147/PHMT.S256309
40.
Mernie G Kloos H Adane M . Prevalence of and Factors Associated With Acute Diarrhea Among Children Under Five in Rural Areas in Ethiopia With and Without Implementation of Community-Led Total Sanitation and Hygiene. BMC Pediatr (2022) 22(1):148. 10.1186/s12887-022-03202-8
41.
Getahun W Adane M . Prevalence of Acute Diarrhea and Water, Sanitation, and Hygiene (WASH) Associated Factors Among Children Under Five in Woldia Town, Amhara Region, Northeastern Ethiopia. BMC Pediatr (2021) 21(1):227. 10.1186/s12887-021-02668-2
42.
Wasihun AG Dejene TA Teferi M Marugán J Negash L Yemane D et al Risk Factors for Diarrhoea and Malnutrition Among Children Under the Age of 5 Years in the Tigray Region of Northern Ethiopia. PLOS ONE (2018) 13(11):e0207743. 10.1371/journal.pone.0207743
43.
Fenta A Alemu K Angaw DA . Prevalence and Associated Factors of Acute Diarrhea Among Under-Five Children in Kamashi District, Western Ethiopia: Community-Based Study. BMC Pediatr (2020) 20(1):236. 10.1186/s12887-020-02138-1
44.
Solomon ET Gari SR Kloos H Mengistie B . Diarrheal Morbidity and Predisposing Factors Among Children Under 5 Years of Age in Rural East Ethiopia. Trop Med Health (2020) 48:66. 10.1186/s41182-020-00253-4
45.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. 10.1136/bmj.n71
46.
World Health Organization. Diarrhoeal Disease (2022). Available from: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (Accessed July 20, 2022).
47.
Joanna Briggs Institute (JBI). Critical-appraisal-Tools - Critical Appraisal Tools (2022). Available from: https://jbi.global/critical-appraisal-tools (Accessed November 28, 2022).
48.
Reeves BC Deeks JJ Higgins JP Shea B Tugwell P Wells GA et al Including Non‐Randomized Studies on Intervention Effects. Cochrane handbook Syst Rev Interventions (2019) 595–620. 10.1002/9781119536604.ch24
49.
DerSimonian R Laird N . Meta-Analysis in Clinical Trials. Control Clin Trials (1986) 7(3):177–88. 10.1016/0197-2456(86)90046-2
50.
Soboksa NE Hailu AB Gari SR Alemu BM . Water Supply, Sanitation and Hygiene Interventions and Childhood Diarrhea in Kersa and Omo Nada Districts of Jimma Zone, Ethiopia: A Comparative Cross-Sectional Study. J Health Popul Nutr (2019) 38(1):45. 10.1186/s41043-019-0205-1
51.
Getachew A Guadu T Tadie A Gizaw Z Gebrehiwot M Cherkos DH et al Diarrhea Prevalence and Sociodemographic Factors Among Under-Five Children in Rural Areas of North Gondar Zone, Northwest Ethiopia. Int J Pediatr (2018) 2018:6031594. 10.1155/2018/6031594
52.
Ayalew AM Mekonnen WT Abaya SW Mekonnen ZA . Assessment of Diarrhea and its Associated Factors in Under-Five Children Among Open Defecation and Open Defecation-Free Rural Settings of Dangla District, Northwest Ethiopia. J Environ Public Health (2018) 2018:4271915. 10.1155/2018/4271915
53.
Degebasa M Dawit Z Marama M . Diarrheal Status and Associated Factors in Under Five Years Old Children in Relation to Implemented and Unimplemented Community-Led Total Sanitation and Hygiene in Yaya Gulele in 2017. Pediatr Health Med Ther (2018) 9:109–21. 10.2147/PHMT.S159366
54.
Wagari S Girma H Geremew A . Water, Sanitation, and Hygiene Service Ladders and Childhood Diarrhea in Haramaya Demographic and Health Surveillance Site, Eastern Ethiopia. Environ Health Insights (2022) 16:11786302221091416. 10.1177/11786302221091416
55.
Kebede FW Berhe Gebremedhin G Gebregergs GB Marama Mokonnon T . Assessment of Poor Home Management Practice of Diarrhea and Associated Factors Among Caregivers of Under-Five Years Children in Urban and Rural Residents of Doba Woreda, Ethiopia: Comparative Cross-Sectional Study. Int J Pediatr (2019) 2019:8345245. 10.1155/2019/8345245
56.
Feleke DG Chanie ES Admasu FT Bahir S Amare AT Abate HK . Two-Week Prevalence of Acute Diarrhea and Associated Factors Among Under Five Years’ Children in Simada Woreda, South Gondar Zone, Northwest Ethiopia, 2021: A Multi-Central Community Based Cross-Sectional Study. Pan Afr Med J (2022) 42:12. 10.11604/pamj.2022.42.12.32599
57.
Megersa S Benti T Sahiledengle B . Prevalence of Diarrhea and its Associated Factors Among Under-Five Children in Open Defecation Free and Non-Open Defecation Free Households in Goba District Southeast Ethiopia: A Comparative Cross-Sectional Study. Clin Mother Child Health (2019) 16(3):324.
58.
Beyene SG Melku AT . Prevalence of Diarrhea and Associated Factors Among Under Five Years Children in Harena Buluk Woreda Oromia Region, South East Ethiopia, 2018. J Public Health Int (2018) 1(2):9–26. 10.14302/issn.2641-4538.jphi-18-2470
59.
Kasye DG Garoma NH Kassa MA . Assessment of the Prevalence of Diarrheal Disease Under-Five Children Serbo Town, Jimma Zone South West Ethiopia. Clin Mother Child Health (2018) 15(1). 10.4172/2090-7214.1000281
60.
Getachew F Tamene M Gesese A Gebremariam S Mezgebe T . Water Handling, Sanitation, and Hygienic Practices and its Association With Under-Five Childhood Diarrhea Among Households of Kirkos Sub City, Addis Ababa, Ethiopia. J Nurs Health C (2022) 7(2):01–7.
61.
Mengistu KD . Prevalence of Diarrhea and Associated Risk Factors Among Under Five Children Visiting Hamusit Health Center, Dera District, South Gondar Zone, Northwest Ethiopia. Master’s thesis. Bahir Dar, Ethiopia: Bahir Dar University (2021).
62.
Damene DA Melkamu BS Yimer HD . Prevalence of Acute Diarrhea and Associated Precipitating Factors Among Under-Five Children in West Guji Zone, Oromia Region, Ethiopia, 2018: Community Based Cross Sectional Study. J Infect Dis Immun (2020) 12(1):1–12. 10.5897/jidi2019.0186
63.
Brhanemeskel H . Assessment of Undernutrition and the Prevalence of Infections, Allergy and Diarrhea Among Bottle-Feed Infants and its Comparison With Non-Bottle Fed Infants Aged 6-23 Months in Bole Sub-City Health Centers, Addis Ababa, Ethiopia: A Case Control Study. Bahir Dar, Ethiopia. Master’s thesis. Bahir Dar, Ethiopia: Bahir Dar University (2021).
64.
Solomon ET Robele S Kloos H Mengistie B . Effect of Household Water Treatment With Chlorine on Diarrhea Among Children Under the Age of Five Years in Rural Areas of Dire Dawa, Eastern Ethiopia: A Cluster Randomized Controlled Trial. Infect Dis Poverty (2020) 9(1):64. 10.1186/s40249-020-00680-9
65.
Uganda Demographic and Health Survey. Uganda Bureau of Statistics Kampala. Uganda Demographic and Health Survey 2016 Key Indicators Report Uganda (2016). Available from: https://dhsprogram.com/pubs/pdf/FR333/FR333.pdf (Accessed July 28, 2022).
66.
Demissie GD Yeshaw Y Aleminew W Akalu Y . Diarrhea and Associated Factors Among Under Five Children in Sub-Saharan Africa: Evidence From Demographic and Health Surveys of 34 Sub-Saharan Countries. PLOS ONE (2021) 16(9):e0257522. 10.1371/journal.pone.0257522
67.
Tareke AA Enyew EB Takele BA . Pooled Prevalence and Associated Factors of Diarrhea Among Under-Five Years Children in East Africa: A Multilevel Logistic Regression Analysis. PLOS ONE (2022) 17(4):e0264559. 10.1371/journal.pone.0264559
68.
Khalil I Colombara DV Forouzanfar MH Troeger C Daoud F Moradi-Lakeh M et al Burden of Diarrhea in the Eastern Mediterranean Region, 1990–2013: Findings From the Global Burden of Disease Study 2013. Am J Trop Med Hyg (2016) 95(6):1319–29. 10.4269/ajtmh.16-0339
69.
Koyuncu A Dufour MSK Watadzaushe C Dirawo J Mushavi A Padian N et al Household Flooring Associated With Reduced Infant Diarrhoeal Illness in Zimbabwe in Households With and Without WASH Interventions. Trop Med Int Health (2020) 25(5):635–43. 10.1111/tmi.13385
70.
Lamberti LM Fischer Walker CL Noiman A Victora C Black RE . Breastfeeding and the Risk for Diarrhea Morbidity and Mortality. BMC Public Health (2011) 11(Suppl. 3):S15. 10.1186/1471-2458-11-S3-S15
71.
Victora CG Bahl R Barros AJD França GVA Horton S Krasevec J et al Breastfeeding in the 21st Century: Epidemiology, Mechanisms, and Lifelong Effect. Lancet (2016) 387(10017):475–90. 10.1016/S0140-6736(15)01024-7
72.
World Health Organization. Breastfeeding (2022). Available from: https://www.who.int/health-topics/breastfeeding (Accessed August 2, 2022).
73.
Nuzhat S Shahunja KM Shahid AS Khan SH Islam SB Islam MR et al Diarrhoeal Children With Concurrent Severe Wasting and Stunting Compared to Severe Wasting or Severe Stunting. Trop Med Int Health (2020) 25(8):928–35. 10.1111/tmi.13446
74.
Nel E . Diarrhoea and Malnutrition. South Afr J Clin Nutr (2010) 23(Suppl. 1):15–8. 10.1080/16070658.2010.11734262
75.
Desmennu AT Oluwasanu MM John-Akinola YO Opeyemi O Ayo AS . Maternal Education and Diarrhea Among Children Aged 0-24 Months in Nigeria. Afr J Reprod Health (2017) 21(3):27–36. 10.29063/ajrh2017/v21i3.2
76.
Solomon ET Gari SR Kloos H Alemu BM . Handwashing Effect on Diarrheal Incidence in Children Under 5 Years Old in Rural Eastern Ethiopia: A Cluster Randomized Controlled Trial. Trop Med Health (2021) 49(26):26. 10.1186/s41182-021-00315-1
77.
Ejemot RI Ehiri JE Meremikwu MM Critchley JA . Cochrane Review: Hand Washing for Preventing Diarrhoea. Evid-based Child Health Cochrane Rev J (2009) 4(2):893–939. 10.1002/ebch.373
78.
Edward A Jung Y Chhorvann C Ghee AE Chege J . Association of Mother’s Handwashing Practices and Pediatric Diarrhea: Evidence From a Multi-Country Study on Community Oriented Interventions. J Prev Med Hyg (2019) 60(2):E93–E102. 10.15167/2421-4248/jpmh2019.60.2.1088
79.
Ejemot-Nwadiaro RI Ehiri JE Arikpo D Meremikwu MM Critchley JA . Hand Washing Promotion for Preventing Diarrhoea. Cochrane Database Syst Rev (2015) 2015(9):CD004265. 10.1002/14651858.CD004265.pub3
80.
Hirai M Roess A Huang C Graham JP . Exploring the Link Between Handwashing Proxy Measures and Child Diarrhea in 25 Countries in Sub-Saharan Africa: A Cross-Sectional Study. J Water Sanit Hyg Dev (2017) 7(2):312–22. 10.2166/washdev.2017.126
81.
Shah D Choudhury P Gupta P Mathew JL Gera T Gogia S et al Promoting Appropriate Management of Diarrhea: A Systematic Review of Literature for Advocacy and Action: UNICEF-PHFI Series on Newborn and Child Health, India. Indian Pediatr (2012) 49(8):627–49. 10.1007/s13312-012-0134-1
82.
Clasen TF Alexander KT Sinclair D Boisson S Peletz R Chang HH et al Interventions to Improve Water Quality for Preventing Diarrhoea. Cochrane Database Syst Rev (2015) 2015(10):CD004794. 10.1002/14651858.CD004794.pub3
83.
Clasen T Schmidt WP Rabie T Roberts I Cairncross S . Interventions to Improve Water Quality for Preventing Diarrhoea: Systematic Review and Meta-Analysis. BMJ (2007) 334(7597):782. 10.1136/bmj.39118.489931.BE
84.
Agustina Dukabain OM Singga S Wanti W Suluh DG Mado FG . Home Sanitation Facilities and Prevalence of Diarrhea for Children in Oelnasi Village, Kupang Tengah Sub-District. Gac Sanit (2021) 35(Suppl. 2):S393–S395. 10.1016/j.gaceta.2021.10.059
85.
Patil SR Arnold BF Salvatore AL Briceno B Ganguly S Colford JM et al The Effect of India’s Total Sanitation Campaign on Defecation Behaviors and Child Health in Rural Madhya Pradesh: A Cluster Randomized Controlled Trial. Plos Med (2014) 11(8):e1001709. 10.1371/journal.pmed.1001709
86.
Clasen T Boisson S Routray P Torondel B Bell M Cumming O et al Effectiveness of a Rural Sanitation Programme on Diarrhoea, Soil-Transmitted Helminth Infection, and Child Malnutrition in Odisha, India: A Cluster-Randomised Trial. Lancet Glob Health (2014) 2(11):e645–53. 10.1016/S2214-109X(14)70307-9
87.
Jung YT Hum RJ Lou W Cheng YL . Effects of Neighbourhood and Household Sanitation Conditions on Diarrhea Morbidity: Systematic Review and Meta-Analysis. PLOS ONE (2017) 12(3):e0173808. 10.1371/journal.pone.0173808
88.
United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development (2022). Available from: https://sdgs.un.org/2030agenda (Accessed September 29, 2022).
89.
Arba A Aydiko E Baza D . Prevalence of Diarrheal Disease Among Under-Five Children in Worabe Town, Southern Ethiopia. Am J Life Sci (2020) 8(4):45. 10.11648/j.ajls.20200804.11
90.
Zegeye Z . Prevalence of Childhood Diarrhea Disease and Associated Factors Among Under Five Children in Slum Area of Metropolitan City, Bahir Dar, Gondar and Dessie in Amhara Region, Ethiopia. Master’s thesis. Bahir Dar, Ethiopia: Bahir Dar University (2021).
91.
Kassie G . Magnitude of Common Childhood Illness, Health Care Seeking Behavior, and Associated Factors in Efratana Gidim District, East Amhara, Ethiopia, 2020. Master’s thesis. Addis Ababa, Ethiopia: Addis Ababa University (2020).
Summary
Keywords
diarrhea, Ethiopia, risk factors, under-five children, WASH
Citation
Sahiledengle B, Atlaw D, Mwanri L, Petrucka P, Kumie A, Tekalegn Y, Desta F, Zenbaba D, Mesfin T, Gomora D and Agho KE (2024) Burden of Childhood Diarrhea and Its Associated Factors in Ethiopia: A Review of Observational Studies. Int J Public Health 69:1606399. doi: 10.3389/ijph.2024.1606399
Received
15 July 2023
Accepted
20 May 2024
Published
05 June 2024
Volume
69 - 2024
Edited by
Jean Tenena Coulibaly, Félix Houphouët-Boigny University, Côte d’Ivoire
Reviewed by
Gaoussou Coulibaly, Félix Houphouët-Boigny University, Côte d’Ivoire
One reviewer who chose to remain anonymous
Updates
Copyright
© 2024 Sahiledengle, Atlaw, Mwanri, Petrucka, Kumie, Tekalegn, Desta, Zenbaba, Mesfin, Gomora and Agho.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biniyam Sahiledengle, biniyam.sahiledengle@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.