Abstract
Objectives: This study examined factors associated with COVID-19 vaccination intention at the very beginning of the vaccination campaign in a representative sample of the population in southern Switzerland.
Methods: In March 2021, we measured vaccination intention, beliefs, attitudes, and trust in a sample of the Corona Immunitas Ticino study.
Results: Of the 2681 participants, 1933 completed the questionnaire (response rate = 72%; 55% female; meanage = 41, SD = 24, rangeage = 5–91). Overall, 68% reported an intention to get vaccinated. Vaccination intention was higher in social/healthcare workers, and increased with age, trust in public health institutions, and confidence in the vaccine efficacy. Prior infection of a family member, predilection for waiting for more evidence on the safety and efficacy of the vaccine, and for alternative protective means were negatively associated with intention.
Conclusion: In view of needs of COVID-19 vaccine boosters and of suboptimal vaccination coverage, our results have relevant public health implications and suggest that communication about vaccine safety and efficacy, and aims of vaccination programs, should be bi-directional, proportionate, and tailored to the concerns, expectations, and beliefs of different population subgroups.
Introduction
Following the approval of the first COVID-19 vaccines by Swissmedic in December 2020, vaccination campaigns began across Switzerland (1). In Ticino, the Italian speaking canton that borders the heavily affected regions in northern Italy, vaccinations began in January 2021 prioritizing older adults and frontline healthcare and social workers (2). In the first quarter of 2021, Switzerland administered, free of charge at the point of delivery (3), the mRNA vaccines from Pfizer-BioNTech and Moderna (4). In mid-March 2021, when the present study was carried out, the 14-day incidence of confirmed cases per 100,000 inhabitants at the national level was 194. In Ticino, this number ranged from 240 to 479 cases (see Figure 1) (5).
FIGURE 1
14-day normalized incidence of COVID-19 laboratory-confirmed cases, Switzerland, Ticino, 15.3.2021, source: https://www.covid19.admin.ch/en/epidemiologic/case.
The availability of COVID-19 vaccines is crucial for protection and to reduce the risk of severe disease through adequate immunization coverage. However, availability alone is not sufficient to achieve these aims (6–10). Vaccination decisions are influenced by several interacting drivers, including emotional, cultural, social, religious, logistical, political, and cognitive factors (11–13). The COVID-19 pandemic entails additional and unique challenges for public confidence in vaccines (14, 15). The development of vaccines was exceptionally fast, data on both safety and efficacy is, to date, short-term. Its use was initially authorized under emergency use terms (EUA) (16), and the composition, functioning and technology of most COVID-19 vaccines are relatively novel (17). Furthermore, concerns about the Oxford-AstraZeneca and Johnson and Johnson vaccine in other countries triggered safety concerns and confusion in the public (14, 18, 19). Another considerable challenge is posed by the infodemic associated with the pandemic and the unprecedented spread of misinformation, which does not spare vaccines (20–22). These factors contribute to vaccine hesitancy, defined as a “delay in acceptance or refusal of vaccination despite availability of (safe and efficacious) vaccines” (11) (p. 4163).
Global COVID-19 vaccination acceptance was ≥70%, but with marked geographic variations (23). Intention to get vaccinated ranged from 28% in Congo to 93% in China during the first year of the pandemic (24, 25). Lower levels of education and working in the healthcare sector were associated with lower intention (26). Some personality traits and attitudes, having received an influenza vaccination in the last year, and perceived threat to physical health were all associated with greater intention (23, 27).
Serological testing has become more common and accessible through large serosurveys used to measure the extent of the COVID-19 infection in populations. However, little is known about the potential modulating effect of known prior infection on vaccination intention. Callaghan et al. (28) found that past infection with COVID-19 was negatively correlated with vaccine uptake. Exposure to the virus conceivably confers protection against re-infection and/or some level of functional immunity against secondary severe COVID-19 disease (29). Therefore, knowledge of immune memory from primary infection(s) may lead to a lower intent to vaccinate against COVID-19. Moreover, evidence on vaccination intention in older adults, who are more prone to develop severe symptoms, and children, for whom vaccines were approved in December 2021, is extremely sparse, but very important (30). Evidence on vaccination intention in teenagers and children, who are often asymptomatic carriers (31), is crucial to inform public health decisions, and may contribute to attaining herd immunity (32, 33).
Aim and Research Questions
The aim of this study was to measure the intention to get vaccinated against COVID-19, to identify the attitudes and beliefs associated with COVID-19 vaccination and to assess their role, together with socio-demographic factors and known prior infection of the self or a family member, in predicting intention during the early phase of the vaccination campaign, in a representative sample of the population of southern Switzerland (Canton Ticino). In 2019 (34), there were 8.6 million inhabitants in Switzerland, of which 20% aged 0–19, 61% aged 20–64, and 19% aged 65 years or older. Canton Ticino had approximately 350′000 inhabitants in 2019, with 18% aged 0–19, 59% aged 20–64, and 23% aged 65 years or older. Foreign nationals constituted 25% of the population at the national level and 28% in Ticino.
The study addresses the following three research questions (RQ):
RQ1: How many individuals are likely or very likely to decide to get vaccinated against COVID-19 among the general population residing in Ticino, stratified by age groups?
RQ2: What are the most common COVID-19 vaccination-related beliefs among the general population residing in Ticino?
RQ3: What is the role of general and COVID-19 vaccination-related beliefs, attitudes, socio-demographic factors, and known prior infection in predicting COVID-19 vaccination intention?
Methods
Study Design, Recruitment, and Procedures
The data for this study come from the prospective cohort, population-based Corona Immunitas Ticino study aiming to assess seroprevalence, and the impact of the COVID-19 pandemic in Ticino.
Between July and September 2020, 13′226 invitation letters were sent to a randomly selected, age stratified sample of residents extracted from the residential registry in Ticino: in July to adults aged 20–64 (n = 4000), and in September to parents of children aged 5–13 (n = 2170), teenagers aged 14–19 (n = 2094), and older adults aged 65+ (n = 4962). Diplomats, people under guardianship/asylum, those with a short-term residence permit, older adults living in long-term facilities were not sampled. Italian speaking participants (or their legal representative) who provided informed consent were enrolled in the digital cohort study upon completion of an online registration form and a baseline questionnaire. We implemented repeated questionnaires in REDCap (35, 36). For children aged 5–13, parents completed the questionnaires with reference to the participating child. Older adults with limited Internet access and digital skills were interviewed by a dedicated interviewer using computer assisted telephone interviewing.
We administered additional ad-hoc surveys to participants in specific phases of the project, including 1) a sero-specific questionnaire at the time of blood collection and 2) a vaccination questionnaire collecting data for the present study. The vaccination questionnaire was sent in March 2021 to participants in the digital cohort (n = 2681). They received a reminder after 2 weeks from the release of the questionnaire. The study was approved by the Cantonal Ethics Committee (2020-01514).
Measures
COVID-19 Vaccination Intention
We measured vaccination intention with the item “Once the coronavirus vaccine is available to you (your child), how likely is it that you will decide to get (your child) vaccinated?” on a 5-point Likert scale ranging from 1 “very unlikely” to 5 “very likely”.
COVID-19 Vaccines and Vaccination Beliefs
We used 22 items from previously validated scales (37–39) and previous studies (14) to measure general and COVID-19-related vaccine and vaccination beliefs. We explored perceived efficacy, perceived safety, and preference for natural immunity (see Supplementary Table S1 in Supplement for a complete list of all items). We scaled responses using a 5-point Likert scale ranging from 1 “strongly disagree” to 5 “strongly agree”.
Attitude and Trust Towards Vaccination
We measured attitude and trust towards vaccination with a 6-item scale, the Vaccination Acceptance Index (VAI) (40), adapted to the Swiss context. The scale includes one item measuring vaccination confidence, and five items on trust, each using a 5-point Likert scale ranging from 1 “strongly disagree” to 5 “strongly agree” (see Supplementary Table S1 in Supplement). Following the proposed procedure, we converted all items to a scale from 0–100 with higher scores indicating higher acceptance levels (41). The scale showed high internal consistency (Cronbach’s alpha = 0.902).
Prior Infection
To assess prior COVID-19 infection, we carried out SARS-CoV-2-IgG antibody testing with a previously validated Luminex assay on sera obtained from peripheral venous blood (42), combined with self-reported positive PCR or serology test results from the baseline and follow-up questionnaires. We also assessed whether participants were aware of a positive PCR or serology test result of a family member. We created two dummy variables, for the participant and the family member, respectively, with 1 indicating “at least one positive test result, either lab-confirmed or reported” and 0 indicating “negative or no test result reported”.
Socio-Demographics
In the baseline questionnaire, we assessed gender, age, highest educational attainment (for participants <20 years, the highest educational attainment of their parents), nationality, number of household members, perceived financial situation and, for participants ≥20 years who indicated to be (self-)employed, whether they worked in the social or healthcare sector, and/or had direct contact with children or youth.
Data Analysis
We used SPSS© v.24 for all statistical analysis. We excluded participants who reported to have already received COVID-19 vaccination (n = 148) and participants who did not answer to at least 50% of the vaccination questionnaire (n = 600). For the remaining analytic sample (n = 1933), we imputed missing values on vaccination intention, beliefs, attitudes, and trust items for 223 (21.5%) respondents using an Expectation-Maximization algorithm. We conducted χ2- and independent samples t-tests to assess differences in socio-demographic characteristics and prior infection between the included and excluded participants. Next, we calculated z-scores to detect outliers, defined as those with a z-score at least 3.5 SD greater than the standardized sample mean (43).
For the main analyses, we conducted an Exploratory Factor Analysis (EFA) of all 22 COVID-19 vaccination belief items to explore the underlying latent constructs and structure. We used maximum likelihood extraction with oblique rotation to allow factors to be correlated among each other (44). We set the Eigenvalue to one. We computed compound scores of all extracted factors averaging items with a factor loading of at least 0.30. Items with a loading of less than 0.30 and items with cross-loadings, i.e., at least 0.30 on more than one factor, were discarded. We tested internal consistency of all identified factors with Cronbach’s alpha, and retained for further analysis only those with an alpha of at least 0.70. Next, we ran zero-order correlations among the retained factors, the VAI, and vaccination intention as a final outcome measure. We conducted one-way ANOVA to compare vaccination intention, the VAI, and the retained factors among the four age groups (children, teenagers, adults, and older adults), and hierarchical regression analyses to test if sociodemographic characteristics, prior SARS-CoV-2 infection, VAI, and retained vaccination belief factors were associated with participants’ vaccination intention across all age groups.
Results
Sample Characteristics
The analytical sample included 1933 of the 2681 invited participants in the digital cohort study (72%). Response rates differed by age: 428 of 576 (74%) parents for their children; 222 of 394 (56%) teenagers; 842 of 1081 (78%) adults; and 441 of 630 (70%) older adults. Among respondents, 55% were female and mean age was 41 years (SD = 24). The modal level of highest educational attainment was apprenticeship/professional school (n = 668; 36%). The majority were Swiss (n = 1670; 87%), 236 (12%) European Union/EFTA, and 11 (0.6%) reported a nationality from another country. About half of the sample (51%) reported that their income was just the necessary to live on, and 50% worked either in the social/health care sector, with children <15 years, or clients or students ≥15 years. See Table 1 for sample characteristics.
TABLE 1
| Respondents vaccination questionnaire(N = 1933) | Non-respondents (N = 748) | χ2-test/t-testh | p | General population in canton ticinoa | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Gendera | 2.145 | 0.143 | ||||||
| Female | 1061 | 54.9 | 386 | 51.7 | 180′350 | 51.3 | ||
| Male | 872 | 45.1 | 360 | 48.3 | 171′141 | 48.7 | ||
| Age,a,b (5+) | M = 40.9 | SD = 23.8 | M = 39.1 | SD = 27.1 | 1.594 | 0.111 | M = 47.16 | SD = 22.48 |
| 5–13 | 428 | 22.1 | 148 | 19.8 | 29′000 | 8.5 | ||
| 14–19 | 222 | 11.5 | 172 | 23.0 | 20′343 | 5.9 | ||
| 20–64 | 842 | 43.6 | 239 | 32.0 | 208′833 | 61.0 | ||
| 65+ | 441 | 22.8 | 189 | 25.3 | 84′041 | 24.6 | ||
| Highest educational attainmentc,d | 52.37 | <0.001 | ||||||
| None/Obligatory school | 70 | 3.7 | 77 | 18.0 | 85′951 | 24.3 | ||
| Apprenticeship/professional school | 668 | 35.5 | 240 | 33.8 | 89′842 | 25.4 | ||
| High school | 327 | 17.4 | 99 | 13.9 | 63′314 | 17.9 | ||
| Higher professional training | 135 | 7.2 | 59 | 8.3 | 47′397 | 13.4 | ||
| University/university of applied sciences | 680 | 36.2 | 236 | 33.2 | 67′205 | 19.0 | ||
| Nationalitya | 22.98 | <0.001 | ||||||
| Swiss | 1670 | 87.1 | 596 | 80.9 | 2554′633 | 72.4 | ||
| EU/EFTA | 236 | 12.3 | 126 | 17.1 | 98′858 | 27.6 | ||
| Other | 11 | 0.6 | 15 | 2.0 | ||||
| Number of household memberse | M = 3.0 | SD = 1.3 | M = 3.1 | SD = 1.4 | 1.88 | 0.060 | — | — |
| 1 | 221 | 11.6 | 79 | 10.8 | 65′301 | 40.0 | ||
| 2 | 549 | 28.8 | 208 | 28.5 | 48′960 | 30.0 | ||
| 3 | 364 | 19.1 | 135 | 18.5 | 23′633 | 14.4 | ||
| 4 | 556 | 29.1 | 179 | 24.6 | 19′028 | 11.6 | ||
| 5 | 172 | 9.0 | 100 | 13.7 | 5268 | 3.2 | ||
| 6+ | 47 | 2.5 | 28 | 3.8 | 1470 | 0.9 | ||
| Perceived financial situation | 24.47 | <0.001 | ||||||
| More than the necessary to live | 802 | 46.4 | 244 | 37.6 | — | — | ||
| Just the necessary to live | 885 | 51.2 | 369 | 56.9 | — | — | ||
| Not enough to live | 43 | 2.5 | 36 | 5.5 | — | — | ||
| Job sectorf | 13.09 | 0.004 | ||||||
| Job in social/healthcare | 95 | 12.8 | 46 | 20.6 | 92′829 | 62.2 | ||
| Job with children <15 years | 43 | 5.8 | 4 | 1.8 | ||||
| Job with clients or students ≥15 years | 233 | 31.5 | 66 | 29.6 | ||||
| Other | 369 | 49.9 | 107 | 48.0 | 56′525 | 37.8 | ||
| Known prior COVID-19 infection (self)g | 26.19 | <0.001 | ||||||
| Positive | 287 | 14.8 | 56 | 7.5 | 32′117 | 9.1 | ||
| Negative/unknown | 1646 | 85.2 | 692 | 92.5 | — | — | ||
| Known prior infection (family member) | 8.54 | 0.003 | ||||||
| Positive | 267 | 13.8 | 137 | 18.3 | — | — | ||
| Negative/unknown | 1666 | 86.2 | 611 | 81.7 | — | — | ||
Sample characteristics, Corona Immunitas Ticino (Switzerland, Ticino, 2021).
Note.
Ticino population: Swiss Federal Statistical Office (FSO), 2020, N = 351′491 individuals.
Age groups at time of random sampling by FSO, in May 2020.
(Non-)respondents aged <20 assessed as “highest educational attainment among parents”.
Ticino population: Structural Survey, FSO, Neuchatel,2017,N=353’709 individuals.
Ticino population: Structural Survey, FSO, Neuchatel,2019,N=163’660 households.
Ticino population: Structural Survey, FSO, Neuchatel, 2010, N = 149′354 individuals active in the labor market.
Ticino population: https://www4.ti.ch/dss/dsp/covid19/home/; total number of positive tests as of 29.4.2021.
When M(SD) is reported, independent samples t-test was applied.
Compared to non-respondents (n = 748), participants had a higher educational level, were in a better financial situation, were more likely to have a Swiss nationality, to work in contact with children, and were less likely to work in the social/healthcare sector. Approximately 15% had a prior infection and 14% were aware of a positive test result of a family member (Table 1).
Compared to official socio-demographics statistics from the Swiss Federal Statistical Office (FSO), older participants in Ticino were slightly underrepresented in the current sample, along with men, while non-Swiss nationals and those with a university degree were overrepresented (Table 1).
Vaccination Intention by Age Group
Two thirds (68%) of participants responded to be either “likely” or “very likely” to get vaccinated. Vaccination intention was higher among older adults (87%), followed by teenagers (69%), adults (67%), and parents who decide for their child (49.5%) (Figure 2).
FIGURE 2
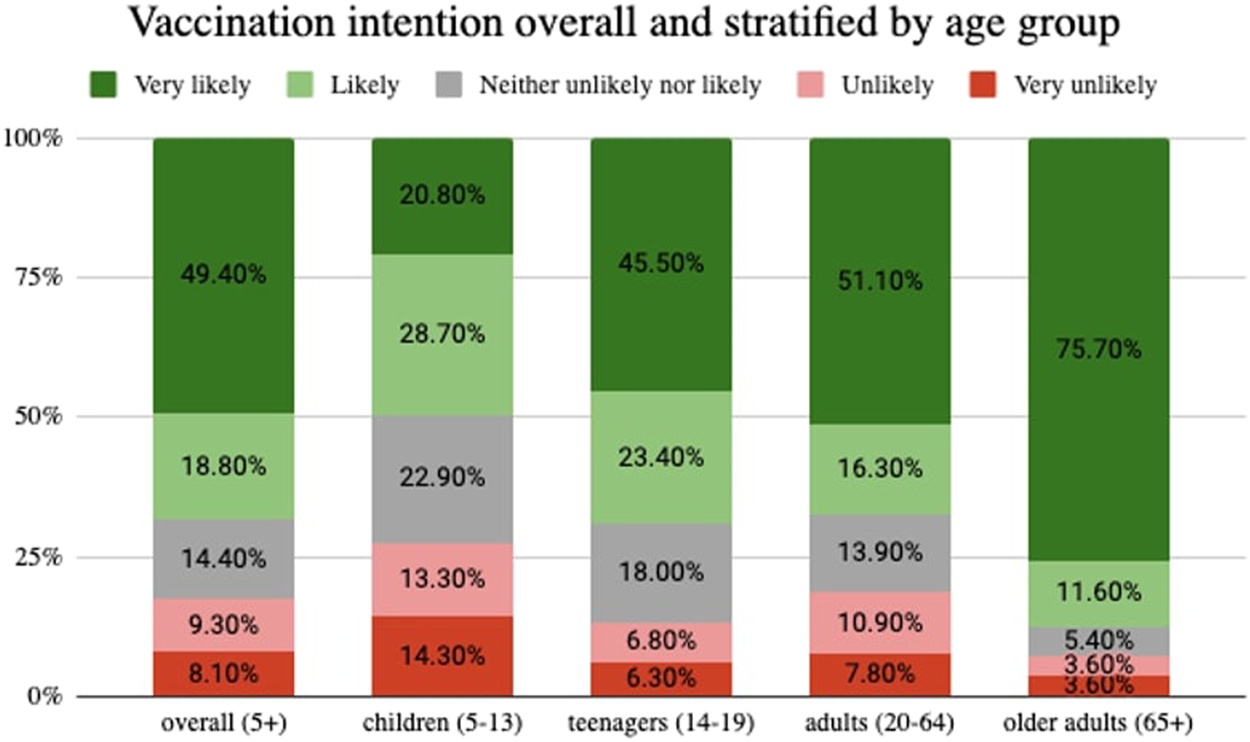
Vaccination intention overall and stratified by age group, Corona Immunitas Ticino (Switzerland, Ticino, 2021).
One-way ANOVA results revealed significant differences in the mean intention score across age groups, except for teenagers and adults, who had similar scores (Table 2).
TABLE 2
| Parents of children (A) | Teenagers (B) | Adults (C) | Older adults (D) | F (3,1929) | Significant mean differencea | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |||
| Vaccination intention | 3.29 | 1.32 | 3.95 | 1.22 | 3.92 | 1.34 | 4.52 | 1.01 | 70.74*** | AB, AC, AD, BD, CD |
| VAI | 51.91 | 15.86 | 55.18 | 14.84 | 51.86 | 17.61 | 58.12 | 16.08 | 16.12*** | AD, BC, CD |
| F1: WaitAndSee | 3.95 | 0.97 | 3.21 | 1.08 | 2.98 | 1.25 | 2.52 | 1.19 | 116.35*** | AB, AC, AD, BC, BD, CD |
| F2: ProtectAndMoveOn | 4.51 | 0.64 | 4.30 | 0.66 | 4.23 | 0.74 | 4.40 | 0.74 | 15.98*** | AB, AC, CD |
| F3: PreferenceForAlternatives | 2.39 | 1.08 | 1.88 | 0.89 | 2.12 | 1.14 | 1.83 | 1.04 | 23.09*** | AB, AC, AD, BC, CD |
| F5: ConfidenceInProtection | 3.33 | 0.89 | 3.58 | 0.87 | 3.47 | 0.93 | 3.86 | 0.85 | 22.60*** | AB, AC, AD, BD, CD |
Oneway ANOVA results for vaccination-related concepts stratified by age group (N = 1933), Corona Immunitas Ticino (Switzerland, Ticino, 2021).
Note: Post hoc comparison based on Tukey HSD; *** p < 0.001.
interpretation: AB, significant difference between group A (parents of children) and B (teenagers), the means presented in the respective columns indicate which of the two groups had a higher average score on the respective concept.
Factor Analytic Results of Vaccination Beliefs
There were 211 outliers for six out of the 22 EFA items. Therefore, we ran the EFA twice (i.e., with and without outliers) and we gauged differences in the obtained pattern matrix. Because the number of factors did not differ in the two models, we included outliers in the final EFA model to capture possible deviant beliefs. We found five factors with Eigenvalues greater than one which, together, explained 58% of the common variance (Supplementary Table S2).
Three items loaded on Factor 1, which indicated a preference for more evidence on the vaccine and concerns about side effects. We thus labelled it “Wait and see.” Four items loaded on Factor 2, conveying a desire to contribute to the protection of one’s self, vulnerable people, and the society at large (“Protect and move on”). Four items loaded on Factor 3, indicating participants’ preference for natural alternatives and skepticism about its development (“Preference for alternatives”). Five items loaded on Factor 4 (“External and medical drivers”) potentially determining vaccination uptake. However, because the internal consistency across the five items was poor (Cronbach’s alpha = 0.504) we excluded Factor 4 from the final regression analysis. Four items loaded on the last factor regarding the belief that the COVID-19 vaccination protects from an infection and transmission of the virus (“Confidence in protection”). Supplementary Table S2 in the Supplement contains a summary of all factor analytic results. Descriptive statistics and zero-order correlations among all factors, vaccination intention, and the VAI are reported in Supplementary Table S3 in the Supplement.
We tested the robustness of our model running the abovementioned analyses twice (i.e., with and without imputed data for missing values). Because the composition and the descriptive results of the retained factors did not differ we used the data with imputations.
One-way ANOVA results showed that parents of children aged 5–13 were more prone to wait and see (Factor 1), to opt for alternatives to the COVID-19 vaccine for their child (once it is available) (Factor 3), and to question the protective value of the vaccine (Factor 5) relative to the other age groups (Table 2).
Predictors of Vaccination Intention
Of the sociodemographics considered in Model 1 of our hierarchical regression analysis, age showed a significant and positive association with vaccination intention, an effect that endured through adjustment Table 3. In Model 2, we added known prior infection to the initial model (one’s own, or that of a family member). Knowing about one’s own past infection was not associated with vaccination intention but knowing about a prior infection of a family member significantly reduced intentions to vaccinate, another effect that endured through adjustment. In Model 3, we added acceptance of and trust in public authorities, measured in the VAI. The VAI was positively and significantly associated with intention, an association that remained significant also in the fully adjusted model (Model 4), where we added our identified factors of vaccination beliefs. The factors “Wait and See” and “Preferences for Alternatives” were significantly and negatively associated with vaccination intention, while beliefs reflecting “Confidence in Protection” were positively associated with intention to vaccinate. In the final model (Model 4), 71% of the overall variance in vaccination intention was explained by the included variables. The VAI (β = 0.309, p < 0.001) was associated with a 30% higher vaccination intention, similar to Factor 3 (“Preference for alternatives”; β = −0.294, p < 0.001), while Factor 5 (“Confidence in protection”; β = 0.182, p < 0.001), and Factor 1 (“Wait and see”; β = −0.143, p < 0.001) were associated with just below 20 and 15% higher vaccination intention, respectively.
TABLE 3
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | p | B | SE | β | p | B | SE | β | p | B | SE | β | p | |
| Intercept | 3.32 | 0.401 | 3.41 | 0.400 | 0.835 | 0.277 | 2.45 | 0.396 | ||||||||
| Gender (female) | −0.242 | 0.140 | — | 0.085 | −0.230 | 0.139 | — | 0.100 | −0.220 | 0.089 | — | 0.014 | −0.133 | 0.076 | — | 0.082 |
| Age | 0.016 | 0.006 | 0.142 | 0.005 | 0.016 | 0.006 | 0.141 | 0.005 | 0.011 | 0.004 | 0.098 | 0.002 | 0.009 | 0.003 | 0.078 | 0.005 |
| Nationality (Swiss)a | −0.063 | 0.214 | — | 0.769 | −0.054 | 0.213 | — | 0.801 | −0.026 | 0.136 | — | 0.846 | −0.017 | 0.116 | — | 0.880 |
| Education (high)b | 0.104 | 0.140 | — | 0.459 | 0.073 | 0.140 | — | 0.603 | −0.046 | 0.089 | — | 0.606 | −0.006 | 0.076 | — | 0.936 |
| Financial situation (more than the necessary to live)c | 0.066 | 0.140 | — | 0.640 | 0.066 | 0.139 | — | 0.636 | −0.089 | 0.089 | — | 0.318 | −0.128 | 0.076 | — | 0.092 |
| Household size | −0.058 | 0.058 | −0.050 | 0.313 | −0.065 | 0.057 | −0.057 | 0.254 | −0.057 | 0.037 | −0.050 | 0.118 | −0.039 | 0.031 | −0.034 | 0.210 |
| Job in social/healthcared | 0.218 | 0.200 | — | 0.277 | 0.221 | 0.199 | — | 0.267 | 0.268 | 0.127 | — | 0.035 | 0.219 | 0.109 | — | 0.045 |
| Job with children <15 yearse | 0.292 | 0.404 | — | 0.470 | 0.343 | 0.402 | — | 0.394 | 0.149 | 0.257 | — | 0.561 | 0.089 | 0.219 | — | 0.686 |
| Known prior infection (self) | 0.017 | 0.173 | — | 0.921 | 0.028 | 0.110 | — | 0.799 | 0.070 | 0.094 | — | 0.458 | ||||
| Known prior infection (family member) | −0.495 | 0.187 | — | 0.008 | −0.307 | 0.120 | — | 0.011 | −0.263 | 0.102 | — | 0.010 | ||||
| Vaccination Acceptance Index (VAI) | 0.056 | 0.002 | 0.757 | <0.001 | 0.023 | 0.003 | 0.309 | <0.001 | ||||||||
| F1: WaitAndSee | −0.152 | 0.040 | −0.143 | <0.001 | ||||||||||||
| F2: ProtectAndMoveOn | 0.086 | 0.055 | 0.049 | 0.117 | ||||||||||||
| F3: PreferenceForAlternatives | −0.352 | 0.050 | −0.294 | <0.001 | ||||||||||||
| F5: ConfidenceInProtection | 0.258 | 0.059 | 0.182 | <0.001 | ||||||||||||
| F statistics | F (8) = 1.85; p = 0.066 | F (10) = 2.20; p = 0.017 | F (11) = 58.28; p <0 .001 | F (15) = 70.03; p <0 .001 | ||||||||||||
| Adjusted R2 | 0.016 | 0.028 | 0.603 | 0.714 | ||||||||||||
Hierarchical linear regression results predicting vaccination intention (N = 1933), Corona Immunitas Ticino (Switzerland, Ticino, 2021).
Significant values.
The data met the assumptions of homogeneity of variance and linearity and the residuals were approximately normally distributed.
Discussion
The aim of this study was to measure vaccination intention and significantly associated vaccine-related beliefs, in addition to socio-demographic factors, attitudes, trust, and prior infection with the SARS-CoV-2 virus in a representative sample of the population in Ticino, Switzerland.
We found that 68% of participants intended to get vaccinated. Of four factors capturing vaccine-related beliefs, three were significantly associated with vaccination intention, namely an inclination to wait for more evidence on safety and efficacy, a preference for alternative protection means, and confidence in the protective role of the vaccine. Moreover, participants wanting to wait for more evidence on the safety and efficacy of the vaccination had a significantly lower intention to get both themselves and their children vaccinated. This finding is in line with one survey of 3′000 Saudi residents, in which the majority of refusers reported they would accept the vaccine if additional studies confirmed safety and effectiveness (45). Evidence from the pre-COVID-19 era showed that lack of and need for more information and a vaccine’s novelty were the most frequently mentioned reasons for not vaccinating their children (46–48). In the present study, parents were significantly more inclined to “wait and see” more about the safety and efficacy of COVID-19 vaccines, and those with a preference for alternative protection means had lower intention to get vaccinated. Previous studies showed that vaccination rates increased when a mask-wearing policy was introduced, and that those who did not accept wearing a mask were more prone to accept vaccination (49). Finally, confidence in the protective role of the vaccine was a predictor of willingness to vaccination uptake both in ours and other studies (50, 51).
We used previously validated scales and conducted our study in a representative sample of the target population. Although previous studies differed by design, that 68% in our study intended to get vaccinated is consistent with similar estimates found in countries such as France, Germany, Canada, Singapore, Sweden, Nigeria (38), Turkey (52), Saudi Arabia (53), United States (27, 54) and United Kingdom (55), but is slightly lower than average acceptance estimates globally (26). Among parents of children 5–13, only 49.5% intend to vaccinate their child once a vaccine is available, while other studies conducted among parents/guardians found that vaccine acceptance was 65% (47) and 70% (55–57).
In our sample, vaccination intention increased with age, which is echoed by previous studies (26, 50, 51). Older adults may have greater concerns for their health compared to younger adults, and may be more prone to treatment and prevention. In Switzerland, older adults were offered the COVID-19 vaccination at the time of data collection. This may have contributed to a high salience of the topic and media coverage stressing the protective role of the vaccine in older populations. However, one study conducted in Saudi Arabia found that younger age was associated with higher acceptance of the vaccination (45). The different role of age in vaccination intention between countries warrants further investigations, and may be explained by geographic, contextual, and cultural differences.
Consistent with previous evidence, we found higher vaccination intentions among healthcare and social workers compared to the general population (55, 58). Yet, some studies found low acceptance rates among nurses (59, 60) and HCWs in general (61). To the best of our knowledge, there is no previous evidence on the relationship between one’s or a relative’s prior infection with the virus and COVID-19 vaccination intention. In this study, prior infection of one’s self was not associated with vaccination intention, but intention was lower among individuals who reported infection of a family member. Participants in our sample may consider infection-acquired and vaccine-acquired immunity distinctly different and may assume they are not mutually exclusive. Some evidence suggests that antibodies against the SARS-CoV-2 might wane over time, but it remains unknown how likely severe re-infection may occur (62). Nonetheless, the media was and still is covering the evidence on declining SARS-CoV-2 antibodies, often ambivalently (63). There may be a low perceived susceptibility to the disease in people with one or more relatives who had a prior infection because they may have presumed to have acquired natural immunity, irrespective of symptoms or testing. If the infected person experienced only mild symptoms their relatives may assume a similar course of the disease if infected, which may also contribute to diminishing the perceived need of personal protection provided by the vaccine.
Furthermore, our results on the association between trust in government and vaccination intention are in line with a recent survey in which respondents reporting higher levels of trust in information from government sources were more likely to accept a COVID-19 vaccine (26). Accordingly, individuals who are skeptical about vaccinations have also reported distrust in science and traditional medicine, with a preference for alternative remedies and prevention strategies (64, 65).
Participants who had lower vaccination intention also reported a preference to employ other protective means and wait until more information becomes available on the effectiveness and safety of vaccines. Our findings reiterate that reasons behind vaccine hesitancy are complex and include more than just an insufficient knowledge (66). To make an appropriate vaccination decision requires “the capacity to obtain, communicate, process, and understand basic health information and services” (67) (p.210), i.e., having an adequate health literacy level (68). This includes competence and skills in finding and discerning information from trustworthy sources, and to appraise, process, and use this information (69).
Practice Implications
Our findings have several implications for practice. First, that a family member’s prior infection is negatively associated with one’s vaccination intention has an important implication for public health messaging. That having a family member with presumed antibodies against SARS-CoV-2 does not confer any protection on others in the household should be timely and effectively communicated to family members of individuals with a positive test. This is important also because new variants continue to emerge for which the antibodies acquired through exposure to the virus may become ineffective, while some types of vaccines can continuously adapt (70). The link between preference for waiting to know more about the vaccination and lower intention needs to be further unpacked, e.g., how much information is “enough” to take an informed decision? In addition, our results suggest that there are population subgroups who may believe that personal protective and social distancing measures confer greater protection than the vaccination. Communication efforts should address target audience’s specific beliefs and go beyond standard official statements and messages that vaccines are safe and effective (71, 72). This can be done, in part, by disseminating messages by locally trusted sources (73), and by maintaining a regular, transparent, bi-directional communication on the vaccination strategy with the public.
Limitations
Some limitations are worth noting. First, issues of directionality because of the cross-sectional design. Vaccination intention may drive certain beliefs and preferences, such as the preference for alternative means of protection. Furthermore, vaccination intention was measured once, and scientific evidence, media coverage on the COVID-19 vaccine, and vaccination campaigns are rapidly changing, likely influencing vaccination intention. Second, potential social desirability bias cannot be excluded, but we intentionally retained outliers in our analyses to avoid a spurious masking of any polarized positions on the topic of the COVID-19 vaccination (74). Third, although response rate was high, self-selection bias is possible. Our sample was over-represented in terms of Swiss nationals, highly educated, and older participants. Participation in population-based research is generally lower among immigrants and higher among individuals of higher socio-economic status (75). The latter may have more trust in science, researchers, and medicine (76), and have altruistic motivations (77–79). However, the finding that more than one third of our participants reported to be either unlikely or unsure about getting vaccinated confirms the importance of learning about the vaccination-related beliefs of individuals from different sociodemographic backgrounds. Fourth, although hesitancy has been found to be higher for specific COVID-19 vaccines, e.g., in case they are manufactured in China (26), or in case of the Johnson and Johnson’s vaccine (80), we did not discriminate between vaccine types but asked to report on the COVID-19 vaccination, in general. At the time of data collection, only mRNA-based vaccines were available in Switzerland. Fifth, the recommendation of COVID-19 vaccines for teenagers 16 years or older, and the age-based priority strategy in the Swiss vaccination campaign at the time of data collection, potentially led to differences in the perceived relevance of the vaccination and the decision for or against vaccination uptake. Future studies on vaccination intention should, thus, be timely and consider the specific context of the eligibility of the study population. Last, the findings of our study may pertain to specific cultural and local features and should be generalized with caution to other regions in Switzerland, and to other countries (81, 82).
Statements
Ethics statement
This study was reviewed and approved by the Ethics Committee of the Canton of Ticino. Where appropriate, written informed consent to participate in this study was provided by participants’ legal guardian/next of kin.
Author contributions
All authors have contributed to the design of the study, development of the questionnaire and its piloting. AC realized the statistical analyses and drafted the results section. AC, MFa, RA, LS, and EA were major contributors in writing the manuscript. MFa and MFi were major contributors in the interpretation of results. All authors have made a substantial contribution to the writing and intellectual content of the article. All authors read and approved the final manuscript for submission to International Journal Public Health.
Funding
The authors declare that this study received funding from and Corona Immunitas, a public-private partnership supported by the Federal Office of Public Health, various cantons, companies, and private individuals in Switzerland. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We thank all study participants for their valuable time in completing the questionnaires.
Conflict of interest
Author LS served on the MSD European Vaccines Advisory Board in 2019.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2022.1604226/full#supplementary-material
References
1.
Swissmedic. Swissmedic grants Authorisation for the First COVID-19 Vaccine in Switzerland (2020). Available from: https://www.swissmedic.ch/swissmedic/en/home/news/coronavirus-covid-19/covid-19-impfstoff_erstzulassung.html (Accessed Apr 30, 2021).
2.
Repubblica e Cantone Ticino. Vaccinazione e prenotazione - COVID19 (2021). Available from: https://www4.ti.ch/dss/dsp/covid19/vaccinazione/vaccinazione-e-prenotazione/(Accessed Dec 2, 2021).
3.
Federal Office of Public Health (FOPH). COVID-19: Switzerland Can Start Vaccinating Vulnerable Groups Already in December (2020). Available from: https://www.bag.admin.ch/bag/en/home/das-bag/aktuell/medienmitteilungen.msg-id-81762.html (Accessed Apr 30, 2021).
4.
VerbekeRLentackerIDe SmedtSCDewitteH. The Dawn of mRNA Vaccines: The COVID-19 Case. J Controlled Release (2021). 333:511–20. 10.1016/j.jconrel.2021.03.043
5.
Federal Office of Public Health (FOPH). COVID-19 Switzerland. Information on the Current Situation, as of 1 December 2021. Available from: https://www.covid19.admin.ch/en/epidemiologic/case?sum=14d_sum (Accessed Dec 2, 2021).
6.
ReintjesRDasEKlemmCRichardusJHKeßlerVAhmadA. "Pandemic Public Health Paradox": Time Series Analysis of the 2009/10 Influenza A/H1N1 Epidemiology, Media Attention, Risk Perception and Public Reactions in 5 European Countries. Plos One (2016). 11(3):e0151258. 10.1371/journal.pone.0151258
7.
JorgensenPMereckieneJCotterSJohansenKTsolovaSBrownC. How Close Are Countries of the WHO European Region to Achieving the Goal of Vaccinating 75% of Key Risk Groups against Influenza? Results from National Surveys on Seasonal Influenza Vaccination Programmes, 2008/2009 to 2014/2015. Vaccine (2015). 36(4):442–52. 10.1016/j.vaccine.2017.12.019
8.
ZürcherKZwahlenMBerlinCEggerMFennerL. Trends in Influenza Vaccination Uptake in Switzerland: Swiss Health Survey 2007 and 2012. Swiss Med Wkly (2019). 149:w14705. 10.4414/smw.2019.14705
9.
European Centre for Disease Prevention and Control (ECDC). Seasonal Influenza Vaccination and Antiviral Use in EU/EEA Member States (2018). Available from: https://www.ecdc.europa.eu/sites/default/files/documents/seasonal-influenza-antiviral-use-2018.pdf (Accessed Apr 30, 2021).
10.
FOPH. InfluenzaNews (2019). Available from: https://www.vaccinarsicontrolinfluenza.ch/files/downloads/09_i_Grippenews_September_19_def_web.pdf (Accessed Apr 30, 2021).
11.
MacDonaldNESAGE Working Group on Vaccine Hesitancy. Vaccine Hesitancy: Definition, Scope and Determinants. Vaccine (2015). 33(34):4161–4. 10.1016/j.vaccine.2015.04.036
12.
BosshardCBühlerGCravioliniJHermannMKrähenbühlD. SRG Corona-Monitor (2021). Available from: https://sotomo.ch/site/wp-content/uploads/2021/02/6.-SRG-Corona-Monitor.pdf (Accessed Apr 30, 2021).
13.
HotezPJNuzhathTColwellB. Combating Vaccine Hesitancy and Other 21st century Social Determinants in the Global Fight against Measles. Curr Opin Virol (2020). 41:1–7. 10.1016/j.coviro.2020.01.001
14.
FaddaMAlbaneseESuggsLS. When a COVID-19 Vaccine Is Ready, Will We All Be Ready for it?Int J Public Health (2020). 65:711–2. 10.1007/s00038-020-01404-4
15.
WiysongeCSNdwandweDRyanJJacaABatouréOAnyaB-PMet alVaccine Hesitancy in the Era of COVID-19: Could Lessons from the Past Help in Divining the Future?Hum Vaccin Immunother (2021). 1–3. 10.1080/21645515.2021.1893062
16.
World Health Organization (WHO). WHO Lists Two Additional COVID-19 Vaccines for Emergency Use and COVAX Roll-Out (2021). Available from: https://www.who.int/news/item/15-02-2021-who-lists-two-additional-covid-19-vaccines-for-emergency-use-and-covax-roll-out (Accessed Apr 30, 2021).
17.
PardiNHoganMJPorterFWWeissmanD. mRNA Vaccines - a new era in Vaccinology. Nat Rev Drug Discov (2018). 17(4):261–79. 10.1038/nrd.2017.243
18.
WHO. Draft Landscape of COVID-19 Candidate Vaccines (2021). Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (Accessed Apr 30, 2021).
19.
WHO. WHO Statement on AstraZeneca COVID-19 Vaccine Safety Signals (2020). Available from: https://www.who.int/news/item/17-03-2021-who-statement-on-astrazeneca-covid-19-vaccine-safety-signals (Accessed Apr 30, 2021).
20.
KataA. A Postmodern Pandora's Box: Anti-vaccination Misinformation on the Internet. Vaccine (2010). 28(7):1709–16. 10.1016/j.vaccine.2009.12.022
21.
MeggetK. Even Covid-19 Can't Kill the Anti-vaccination Movement. BMJ (2020). 369:m2184. 10.1136/bmj.m2184
22.
WangHLiYHutchMNaidechALuoY. Using Tweets to Understand How COVID-19-Related Health Beliefs Are Affected in the Age of Social Media: Twitter Data Analysis Study. J Med Internet Res (2021). 23(2):e26302. 10.2196/26302
23.
WangQYangLJinHLinL. Vaccination against COVID-19: A Systematic Review and Meta-Analysis of Acceptability and its Predictors. Prev Med (2021). 150:106694. 10.1016/j.ypmed.2021.106694
24.
Al-AmerRManezeDEverettBMontayreJVillarosaARDwekatEet alCOVID-19 Vaccination Intention in the First Year of the Pandemic: A Systematic Review. J Clin Nurs (2021). 31:62–86. 10.1111/jocn.15951
25.
WakeAD. The Willingness to Receive COVID-19 Vaccine and its Associated Factors: "Vaccination Refusal Could Prolong the War of This Pandemic" - A Systematic Review. Rmhp (2021). Vol. 14:2609–23. 10.2147/RMHP.S311074
26.
SchwarzingerMWatsonVArwidsonPAllaFLuchiniS. COVID-19 Vaccine Hesitancy in a Representative Working-Age Population in France: a Survey experiment Based on Vaccine Characteristics. Lancet Public Health (2021). Vol. 6:e210–e221. S2468266721000128. 10.1016/s2468-2667(21)00012-8
27.
ReiterPLPennellMLKatzML. Acceptability of a COVID-19 Vaccine Among Adults in the United States: How many People Would Get Vaccinated?Vaccine (2020). 38(42):6500–7. 10.1016/j.vaccine.2020.08.043
28.
CallaghanTMoghtaderiALueckJAHotezPStrychUDorAet alCorrelates and Disparities of Intention to Vaccinate against COVID-19. Soc Sci Med (2021). 272:113638. 10.1016/j.socscimed.2020.113638
29.
DanJMMateusJKatoYHastieKMYuEDFalitiCEet alImmunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection (2021). Available from: https://science.sciencemag.org/content/371/6529/eabf4063 (Accessed Apr 30, 2021). 10.1126/science.abf4063
30.
MahaseE. Covid Vaccine Could Be Rolled Out to Children by Autumn. BMJ (2021). 372:n723. 10.1136/bmj.n723
31.
BhopalSSBagariaJOlabiBBhopalR. Children and Young People Remain at Low Risk of COVID-19 Mortality. Lancet Child Adolesc Health (2021). 5(5):e12–e13. 10.1016/S2352-4642(21)00066-3
32.
KelvinAAHalperinS. COVID-19 in Children: the Link in the Transmission Chain. Lancet Infect Dis (2020). 20(6):633–4. 10.1016/S1473-3099(20)30236-X
33.
Swiss National COVID-19 Science Task Force. The Role of Children (≤12 Years of Age) and Adolescents (13-17 Years of Age) in the SARS-CoV-2 Pandemic: A Rapid Review (2021). Available from: https://sciencetaskforce.ch/en/policy-brief/the-role-of-children-and-adolescents-0-18-years-of-age-in-the-transmission-of-sars-cov-2-a-rapid-review-09-04-2021/(Accessed Apr 30, 2021).
34.
Federal Statistical Office. Ticino. Available from: https://www.bfs.admin.ch/bfs/en/home/statistics/regional-statistics/regional-portraits-key-figures/cantons/ticino.html (Accessed Dec 2, 2021).
35.
HarrisPATaylorRThielkeRPayneJGonzalezNCondeJG. Research Electronic Data Capture (REDCap)-A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J Biomed Inform (2009). 42(2):377–81. 10.1016/j.jbi.2008.08.010
36.
HarrisPATaylorRMinorBLElliottVFernandezMO'NealLet alThe REDCap Consortium: Building an International Community of Software Platform Partners. J Biomed Inform (2019). 95:103208. 10.1016/j.jbi.2019.103208
37.
LazarusJVRatzanSCPalayewAGostinLOLarsonHJRabinK. A Global Survey of Potential Acceptance of a COVID-19 Vaccine. Nat Med (2020). 20:1–4. 10.1038/s41591-020-1124-9
38.
Neumann-BöhmeSVargheseNESabatIBarrosPPBrouwerWvan ExelJet alOnce We Have it, Will We Use it? A European Survey on Willingness to Be Vaccinated against COVID-19. Eur J Health Econ (2020). 21(7):977–82. 10.1007/s10198-020-01208-6
39.
LarsonHJSchulzWSTuckerJDSmithDMD. Measuring Vaccine Confidence: Introducing a Global Vaccine Confidence index. PLOS Curr (2015). 7. ecurrents.outbreaks.ce0f6177bc97332602a8e3fe7d7f7cc4. 10.1371/currents.outbreaks.ce0f6177bc97332602a8e3fe7d7f7cc4
40.
EllingsonMKSevdalisNOmerSBThomsonA. Validation of the Vaccine Trust Indicator (VTI) in a Multi-Country Survey of Adult Vaccine Attitudes. (unpublished document).
41.
FriemelTNGeberS. The Role of Socio-Demographics in Covid-19 Prevention (2021). Available from: https://covid-norms.ch/wp-content/uploads/2021/03/Covid-Norms_Fachgespraech_20210112_Friemel-Geber_webseite.pdf (Accessed Apr 30, 2021).
42.
FenwickCCroxattoACosteATPojerFAndréCPellatonCet alChanges in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J Virol (2021). 95(3). 10.1128/JVI.01828-20
43.
IglewiczBHoaglinD. How to Detect and Handle Outliers. American Society for Quality Control (1993). p. 85.
44.
CostelloABOsborneJ. Best Practices in Exploratory Factor Analysis: Four Recommendations for Getting the Most from Your Analysis. Pract Assess Res Eval (2005). 10(1):7.
45.
MagadmiRMKamelFO. Beliefs and Barriers Associated with Covid-19 Vaccination Among the General Population in Saudi Arabia. BMC Public Health (2021). 21(1):1438. 10.1186/s12889-021-11501-5
46.
FournetNMollemaLRuijsWLHarmsenIAKeckFDurandJYet alUnder-vaccinated Groups in Europe and Their Beliefs, Attitudes and Reasons for Non-vaccination; Two Systematic Reviews. BMC Public Health (2018). 18(1):196. 10.1186/s12889-018-5103-8
47.
GoldmanRDYanTDSeilerMParra CotandaCBrownJCKleinEJet alCaregiver Willingness to Vaccinate Their Children against COVID-19: Cross Sectional Survey. Vaccine (2020). 38(48):7668–73. 10.1016/j.vaccine.2020.09.084
48.
SkjefteMNgirbabulMAkejuOEscuderoDHernandez-DiazSWyszynskiDFet alCOVID-19 Vaccine Acceptance Among Pregnant Women and Mothers of Young Children: Results of a Survey in 16 Countries. Eur J Epidemiol (2021). 36(2):197–211. 10.1007/s10654-021-00728-6
49.
DorriboVLazor-BlanchetCHugliOZanettiG. Health Care Workers’ Influenza Vaccination: Motivations and Mandatory Mask Policy. Occup Med Oxf Engl (2015). 65(9):739–45. 10.1093/occmed/kqv116
50.
GuidryJPDLaestadiusLIVragaEKMillerCAPerrinPBBurtonCWet alWillingness to Get the COVID-19 Vaccine with and without Emergency Use Authorization. Am J Infect Control (2021). 49(2):137–42. 10.1016/j.ajic.2020.11.018
51.
LiuDLuoLXieFYuZMaZFWangYet alFactors Associated with the Willingness and Acceptance of SARS-CoV-2 Vaccine from Adult Subjects in China. Hum Vaccin Immunother (2021). 17(8):1–10. 10.1080/21645515.2021.1899732
52.
SalaliGDUysalMS. COVID-19 Vaccine Hesitancy Is Associated with Beliefs on the Origin of the Novel Coronavirus in the UK and Turkey. Psychol Med (2020). 19:1–3. 10.1017/s0033291720004067
53.
Al-MohaithefMPadhiBK. Determinants of COVID-19 Vaccine Acceptance in Saudi Arabia: A Web-Based National Survey. J Multidiscip Healthc (2020). 13:1657–63. 10.2147/jmdh.s276771
54.
MalikAAMcFaddenSMElharakeJOmerSB. Determinants of COVID-19 Vaccine Acceptance in the US. EClinicalMedicine (2020). 26:100495. 10.1016/j.eclinm.2020.100495
55.
ShermanSMSmithLESimJAmlôtRCuttsMDaschHet alCOVID-19 Vaccination Intention in the UK: Results from the COVID-19 Vaccination Acceptability Study (CoVAccS), a Nationally Representative Cross-Sectional Survey. Hum Vaccin Immunother (2020). 1–10. 10.1080/21645515.2020.1846397
56.
BellSClarkeRMounier-JackSWalkerJLPatersonP. Parents’ and Guardians’ Views on the Acceptability of a Future COVID-19 Vaccine: A Multi-Methods Study in England. Vaccine (2020). 38(49):7789–98. 10.1016/j.vaccine.2020.10.027
57.
RhodesAHoqMMeaseyM-ADanchinM. Intention to Vaccinate against COVID-19 in Australia. Lancet Infect Dis (2021). 21(5):e110. 10.1016/S1473-3099(20)30724-6
58.
YurttasBPoyrazBCSutN. Willingness to Get the COVID-19 Vaccine Among Patients with Rheumatic Diseases, Healthcare Workers and General Population in Turkey: a Web-Based Survey. Rheumatol Int (2021). 41(6):1105–14. 10.1007/s00296-021-04841-3
59.
KwokKOLiK-KWeiWITangAWongSYSLeeSS. Influenza Vaccine Uptake, COVID-19 Vaccination Intention and Vaccine Hesitancy Among Nurses: A Survey. Int J Nurs Stud (2021). 114:103854. 10.1016/j.ijnurstu.2020.103854
60.
DrorAAEisenbachNTaiberSMorozovNGMizrachiMZigronAet alVaccine Hesitancy: the Next challenge in the Fight against COVID-19. Eur J Epidemiol (2020). 35(8):775–9. 10.1007/s10654-020-00671-y
61.
Kabamba NzajiMKabamba NgombeLNgoie MwambaGBanza NdalaDBMbidi MiemaJLuhata LungoyoCet alAcceptability of Vaccination against Covid-19 Among Healthcare Workers in the Democratic Republic of the Congo. Pragmatic Obs Res (2020). 11:103–9. 10.2147/por.s271096
62.
ChiaWNZhuFOngSWXYoungBEFongS-WLe BertNet alDynamics of SARS-CoV-2 Neutralising Antibody Responses and Duration of Immunity: A Longitudinal Study. Lancet Microbe (2021). 10.1016/S2666-5247(21)00025-2
63.
SelfWH. Decline in SARS-CoV-2 Antibodies after Mild Infection Among Frontline Health Care Personnel in a Multistate Hospital Network — 12 States, April–August 2020. MMWR Morb Mortal Wkly Rep (2020). 69(47):1762–6. 10.15585/mmwr.mm6947a2
64.
HornseyMJLoberaJDíaz-CatalánC. Vaccine Hesitancy Is Strongly Associated with Distrust of Conventional Medicine, and Only Weakly Associated with Trust in Alternative Medicine. Soc Sci Med (2020). 255:113019. 10.1016/j.socscimed.2020.113019
65.
JustwanFBaumgaertnerBCarlisleJECarsonEKizerJ. The Effect of Trust and Proximity on Vaccine Propensity. Plos One (2019). 14(8):e0220658. 10.1371/journal.pone.0220658
66.
BiasioLR. Vaccine Literacy Is Undervalued. Hum Vaccin Immunother (2019). 15(11):2552–3. 10.1080/21645515.2019.1609850
67.
RatzanSC. Health Literacy: Communication for the Public Good. Health Promot Int (2001). 16(2):207–14. 10.1093/heapro/16.2.207
68.
BiasioLR. Vaccine Hesitancy and Health Literacy. Hum Vaccin Immunother (2016). 13(3):701–2. 10.1080/21645515.2016.1243633
69.
RatzanSC. Vaccine Literacy: A New Shot for Advancing Health. J Health Commun (2011). 16(3):227–9. 10.1080/10810730.2011.561726
70.
DarbyACHiscoxJA. Covid-19: Variants and Vaccination. BMJ (2021). 372:n771. 10.1136/bmj.n771
71.
ThomsonAVallée-TourangeauGSuggsLS. Strategies to Increase Vaccine Acceptance and Uptake: From Behavioral Insights to Context-specific, Culturally-Appropriate, Evidence-Based Communications and Interventions. Vaccine (2018). 36(44):6457–8. 10.1016/j.vaccine.2018.08.031
72.
SuggsLSMcIntyreC. Are We There yet? an Examination of Online Tailored Health Communication. Health Educ Behav (2009). 36(2):278–88. 10.1177/1090198107303309
73.
GermaniFBiller-AndornoN. The Anti-vaccination Infodemic on Social media: A Behavioral Analysis. Plos One (2021). 16(3):e0247642. 10.1371/journal.pone.0247642
74.
SchmidtALZolloFScalaABetschCQuattrociocchiW. Polarization of the Vaccination Debate on Facebook. Vaccine (2018). 36(25):3606–12. 10.1016/j.vaccine.2018.05.040
75.
PurdieDMDunneMPBoyleFMCookMDNajmanJM. Health and Demographic Characteristics of Respondents in an Australian National Sexuality Survey: Comparison with Population Norms. J Epidemiol Community Health (2002). 56(10):748–53. 10.1136/jech.56.10.748
76.
SlegersCZionDGlassDKelsallHFritschiLBrownNet alWhy Do People Participate in Epidemiological Research?J Bioethical Inq (2015). 12(2):227–37. 10.1007/s11673-015-9611-2
77.
CarreraJSBrownPBrodyJGMorello-FroschR. Research Altruism as Motivation for Participation in Community-Centered Environmental Health Research. Soc Sci Med (2018). 196:175–81. 10.1016/j.socscimed.2017.11.028
78.
BrowneJLReesCODelden Jjm vanAgyepongIGrobbeeDEEdwinAet alThe Willingness to Participate in Biomedical Research Involving Human Beings in Low- and Middle-Income Countries: A Systematic Review. Trop Med Int Health (2019). 24(3):264–79. 10.1111/tmi.13195
79.
FiordelliMFaddaMAmatiRAlbaneseE. Older Adults’ Motivations to Participate or Not in Epidemiological Research. Qualitative Inquiry on a Study into Dementia in Switzerland. Plos One (2021). 16(2):e0247141. 10.1371/journal.pone.0247141
80.
LovelaceBBreuningerK. Panicked Patients Call Doctors as Covid Vaccine Hesitancy Rises with J&J Blood Clot Issue (2021). Available from https://www.cnbc.com/2021/04/13/more-people-likely-wont-want-jjs-covid-vaccine-following-latest-issues-with-blood-clots.html (Accessed Apr 30, 2021).
81.
HeadKJKastingMLSturmLAHartsockJAZimetGD. A National Survey Assessing SARS-CoV-2 Vaccination Intentions: Implications for Future Public Health Communication Efforts. Sci Commun (2020). 42(5):698–723. 10.1177/1075547020960463
82.
DetocMBruelSFrappePTardyBBotelho-NeversEGagneux-BrunonA. Intention to Participate in a COVID-19 Vaccine Clinical Trial and to Get Vaccinated against COVID-19 in France during the Pandemic. Vaccine (2020). 38(45):7002–6. 10.1016/j.vaccine.2020.09.041
Summary
Keywords
vaccine hesitancy, pandemic, vaccination, Switzerland, COVID-19
Citation
Fadda M, Camerini AL, Fiordelli M, Corna L, Levati S, Amati R, Piumatti G, Crivelli L, Suggs LS and Albanese E (2022) Why Vaccinate Against COVID-19? A Population-Based Survey in Switzerland. Int J Public Health 67:1604226. doi: 10.3389/ijph.2022.1604226
Received
30 April 2021
Accepted
24 February 2022
Published
23 March 2022
Volume
67 - 2022
Edited by
Machteld Wyss-van den Berg, Swiss Tropical and Public Health Institute (Swiss TPH), Switzerland
Reviewed by
Shingai Machingaidze, European and Developing Countries Clinical Trials Partnership, Netherlands
Updates
Copyright
© 2022 Fadda, Camerini, Fiordelli, Corna, Levati, Amati, Piumatti, Crivelli, Suggs and Albanese.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta Fadda, marta.fadda@usi.ch
†These authors share first authorship
This Original Article is part of the IJPH Special Issue “Vaccination in the COVID-19 Pandemic”
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.